Adverse reactions and anti-Covid vaccines. Aifa report
Anti-Covid vaccines: 56 thousand adverse events out of 18 million doses. 91% of the reports referred to non-serious events. 34 thrombosis cases registered in Italy. All the details of the fourth AIFA pharmacovigilance report
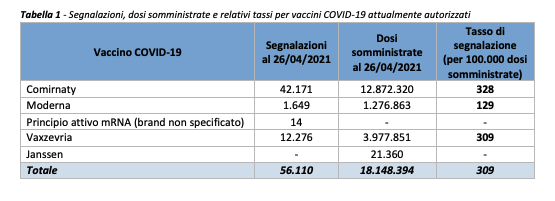
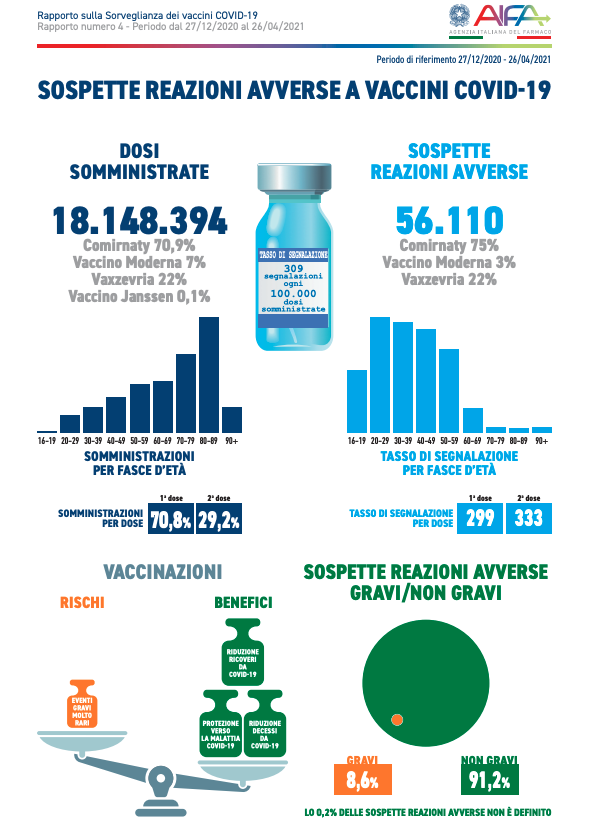
From December 27 to April 26, out of a total of 18,148,394 doses administered, 56,110 reports of adverse events were received following the inoculation of the four approved vaccines: Moderna, Pfizer, AstraZeneca and Johnson & Johnson.
And serious reports correspond to 8.6% of the total, with a rate of 27 serious events per 100,000 doses administered, regardless of the type of vaccine, the dose (first or second) and the possible causal role of vaccination. This is what emerges from the fourth Pharmacovigilance Report on Covid-19 vaccines, published by the Italian Medicines Agency (Aifa).
According to the study, 91% of reports refer to non-serious, completely resolving events, such as injection site pain, fever, asthenia / fatigue, muscle aches.
“The benefit risk analysis – commented with Sole 24 Ore Massimo Andreoni, director of UOC Infectious Diseases of the University of Rome Tor Vergata – remains absolutely in favor of vaccines. Furthermore, the data does not show that there is a vaccine that is more dangerous than others ”.
All the details on the fourth report on vaccine surveillance by Aifa.
REPORTS OF ADVERSE EVENTS
The resulting reporting rate, the report reads, is 309 per 100,000 doses, of which 91% refer to non-serious events, which resolve completely, such as pain at the injection site, fever, asthenia / fatigue, muscle aches. .
"As reported in previous Reports, the reported events occur mostly on the same day of vaccination or the next day (85% of cases)".
SERIOUS ONES 8.6% OF THE TOTAL
Serious reports correspond to 8.6% of the total, with a rate of 27 serious events per 100,000 doses administered, regardless of the type of vaccine, the dose (first or second) and the possible causal role of the vaccination.
WHEN IS A REPORT CONSIDERED SERIOUS?
“An event is always considered serious if it involves hospitalization, first aid, immediate life threatening, disability, congenital anomalies, death, other clinically relevant condition. Furthermore, some adverse events are considered serious regardless of the clinical consequences if present in a list published and periodically updated by the European Medicines Agency, under the name of IME list (Important Medical Events). Based on these criteria, p can be considered serious. ex. a fever ≥ 39 ° which may require the administration of a drug ”, explains Aifa.
MOST REPORTS ARE RELATED TO THE PFIZER VACCINE
Most of the reports are related to the Comirnaty vaccine (75%), so far the most used in the vaccination campaign (70.9% of the doses administered). And only to a lesser extent to the Vaxzevria vaccine – formerly Covid-19 vaccine AstraZeneca – (22%) and to the Moderna vaccine (3%). On the other hand, in the period considered, there are no reports relating to Covid-19 Vaccine Janssen (0.1% of the doses administered).
THROMBOSIS FOLLOWING THE ASTRAZENECA VACCINE
As regards thrombosis following the AstraZeneca vaccine, the note continues, the reports are in line with those of the rest of Europe.
"In Italy, up to 26 April 2021, 29 reports of intracranial venous thrombosis have been included in the National Pharmacovigilance Network – writes Aifa – and 5 cases of venous thrombosis in atypical sites".
“The mean time to onset was approximately 8 days after administration of the 1st dose of the Vaxzevria vaccine. Overall, in about 53% of cases, the thrombotic event is associated with a reduction in the total number of platelets. ".
VERY INTENSE HEADACHE THE MOST COMMON SYMPTOM
“In most cases of intracranial venous thrombosis – specifies Aifa -, the onset symptom was headache, more often widespread, very intense and associated with at least one other symptom including general malaise, drowsiness and vomiting. The cases of venous thrombosis in an atypical site, on the other hand, only involved the abdominal venous structures (mesenteric vein, portal vein, etc.) with a clinical presentation characterized mainly by important gastrointestinal symptoms (especially abdominal pain) ".
65% OF THE CASES CONCERNED WOMEN
"Most of these events (22 cases, 65%) involved women with an average age of about 48 years" points out Aifa. “Only in 1/3 of the cases are men (12 cases, 35%) with an average age of about 52 years. The mean time to onset was approximately 8 days after administration of the 1st dose of the Vaxzevria vaccine ”.
CASES WITH FATAL OUTCOME
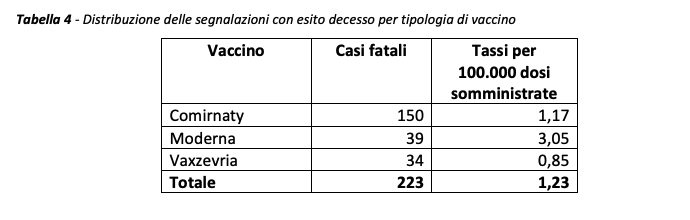
“In Italy, as of April 26, 2021, 223 reports with a“ death ”outcome have been entered, with a reporting rate of cases with a fatal outcome of 1.23 / 100,000 doses for all vaccines. 55.6% of cases concern women, 43.4% men while 0.89% (2 cards) do not report this data ”writes Aifa.
The mean age is 79.1 ± 15.9 years (range 26-104 years).
Aifa specifies that it should be taken into consideration that the Comirnaty (Pfizer) vaccine is the most administered vaccine, in a population more heterogeneous in age than the Moderna vaccine, and, therefore, has the greatest weight in the calculation of the rate of death reports of the entire vaccinated population. In addition, the Moderna vaccine was administered above all to older or very frail patients, with a consequent greater probability of coincident fatal events. Finally, a younger and less fragile population received the Vaxzevria vaccine over the period under review, resulting in a lower reporting rate of deaths.
RATES IN LINE WITH THOSE REPORTED AT INTERNATIONAL LEVEL
The reporting rates observed in Italy are in line with those reported internationally, especially by the United Kingdom Regulatory Agency (MHRA – Medicines and Healthcare products Regulatory Agency), which has extensively used and monitored three of the four vaccines currently administered on our territory.
"According to data updated to April 29, 2021, in fact, the reporting rate of fatal events in the United Kingdom, regardless of causation, corresponds to 1.9 / 100,000 doses administered for the Pfizer / BioNTech vaccine (347 reports for 18 million doses administered), 3.1 / 100,000 for the AstraZeneca vaccine (685 reports for 26.4 million doses), 2 / 100,000 for the Moderna vaccine (2 for 0.1 million doses) ”.
OUT OF 223 REPORTS WITH DEATH OUTCOME, 191 RELATING TO FRAGILE PATIENTS
Aifa specifies that "out of 223 total cases reported, 191 reports are related to frail patients with one or more existing or previous pathologies and in polytherapy, especially cardiovascular diseases, metabolic diseases, oncological diseases, neurodegenerative diseases (Alzheimer's disease), respiratory diseases, severe kidney or liver diseases and diseases of the blood and lymphatic system ".
3 REPORTS RESULTS RELATED TO VACCINATION
Finally, there are 3 death reports related to vaccination. Of these a case already described in the previous Report following the mRna vaccine (relating to a 79-year-old man, with a clinical history of arterial hypertension, previous intervention for triple bypass coronary artery disease and pacemaker implantation, moderate to severe heart failure, monoclonal gammopathy of undetermined significance, prostatic hypertrophy and retinopathy.
And the two new cases refer to a 46-year-old man and a 32-year-old woman who died 12 days after the administration of the 1st dose of Vaxzevria vaccine due to thrombotic events and concomitant thrombocytopenia.
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/reazioni-avverse-e-vaccini-anti-covid-report-aifa/ on Tue, 11 May 2021 10:54:38 +0000.
