Adverse Effects of Covid Vaccines. Ema report
What emerges from the European database of reports of suspected adverse drug reactions, an online portal launched by the EMA
The introduction of green passes to allow a normal return to sociability is a great incentive for all skeptics to get vaccinated as soon as possible.
Among the major concerns are the possible adverse effects , even among those who do not belong to the ranks of the no vax. It should be remembered that the EMA judged the four vaccines administered throughout Europe to be safe and recently extended the Spikevax (Moderna) vaccine to children between 12 and 17 years of age as well .
Pharmacovigilance in Europe
Pharmacovigilance in Europe is ensured by EudraVigilance , the system manages and analyzes information on suspected adverse reactions to medicines authorized or under study in the European Economic Area (EEA). The European Medicines Agency (EMA) manages EudraVigilance on behalf of the European Union (EU) medicines regulatory network.
What is the European database of suspected adverse reaction reports
The European database of suspected adverse drug reaction reports is an online portal, launched by the European Medicines Agency in 2012, which offers public access to reports of suspected adverse drug reactions . Patients, consumers and healthcare professionals report suspected side effects to the national medicines regulatory authorities or to the pharmaceutical company that holds the medicine's marketing authorization. These reports are then transmitted electronically to EudraVigilance , a supranational system that collects reports of suspected side effects. These reports are used to assess the benefits and risks of medicines during the development phase and to monitor their safety after they have been authorized in the European Economic Area (EEA).
Adverse reactions of the four vaccines in Europe
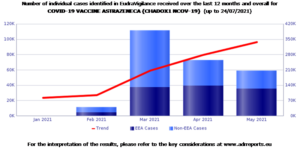
The database, as of July 24, 2021, collected 337,712 reports of adverse events of the Astrazeneca vaccine. Most of the reports, 72%, come from women.
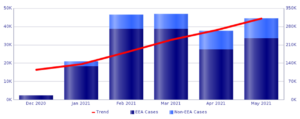
In second place, in terms of the number of reports, there is the Pfizer vaccine with 311,364 reports . Also in this case, most of the reports, 73.2%, concern women.
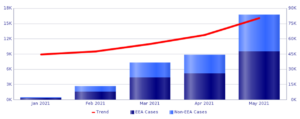
In third place Spikevax, from Moderna , for which the database collected 80,420 reports , 69.5% of which appeared in women.
Finally, there is Johnson & Johnson's Janssen vaccine. In the database, as of 24 July 2021, 18,744 reports of adverse events of the vaccine were recorded, also in this case, most of the reports, 63.2%, come from women.
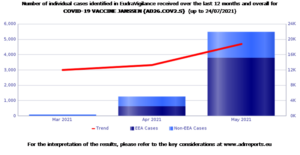
Data on adverse reactions in Italy
From the sixth Pharmacovigilance Report on Covid-19 vaccines, published by the Italian Medicines Agency (AIFA), it emerges that out of a total of "49,512,799 doses administered in Italy, 76,206 reports were received ( reporting rate of 154 per 100,000 doses) , of which 87.9% related to non-serious events, such as pain at the injection site, fever, asthenia / fatigue, muscle aches. Serious reports correspond to 11.9% of the total , with a rate of 18 serious events per 100,000 doses administered, regardless of the type of vaccine, the dose (first or second) and the possible causal role of vaccination " . The data collected concern the reports registered in the National Pharmacovigilance Network between 27 December 2020 and 26 June 2021 for the four vaccines in use in the vaccination campaign.
" Most of the reports – reads the Report – are related to the Comirnaty vaccine (69%)" which is also the most used vaccine in the vaccination campaign with 70.6% of the doses administered, secondly there is Vaxzevria, formerly Astrazeneca with " 24.7% of the reports and 17.3% of the doses administered ", then the Spikevax vaccine ( Moderna) with "5.2% of the reports and 9.6% of the doses administered" and finally the Covid vaccine- 19 by Johnson & Johnson Janssen with only “1.1% of reports and 2.5% of doses administered”.
The most common adverse events
According to the AIFA report, the most reported adverse events are "fever, fatigue, headache, muscle / joint pain, injection site pain, chills and nausea". Thus flu-like symptoms that occurred more frequently " after the second dose of mRNA vaccines and after the first dose of Vaxzevria ". In most cases, the reaction occurred "on the same day as the vaccination or the next day and only more rarely beyond the following 48 hours".
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/effetti-avversi-dei-vaccini-anti-covid-report-ema/ on Wed, 28 Jul 2021 04:36:58 +0000.
