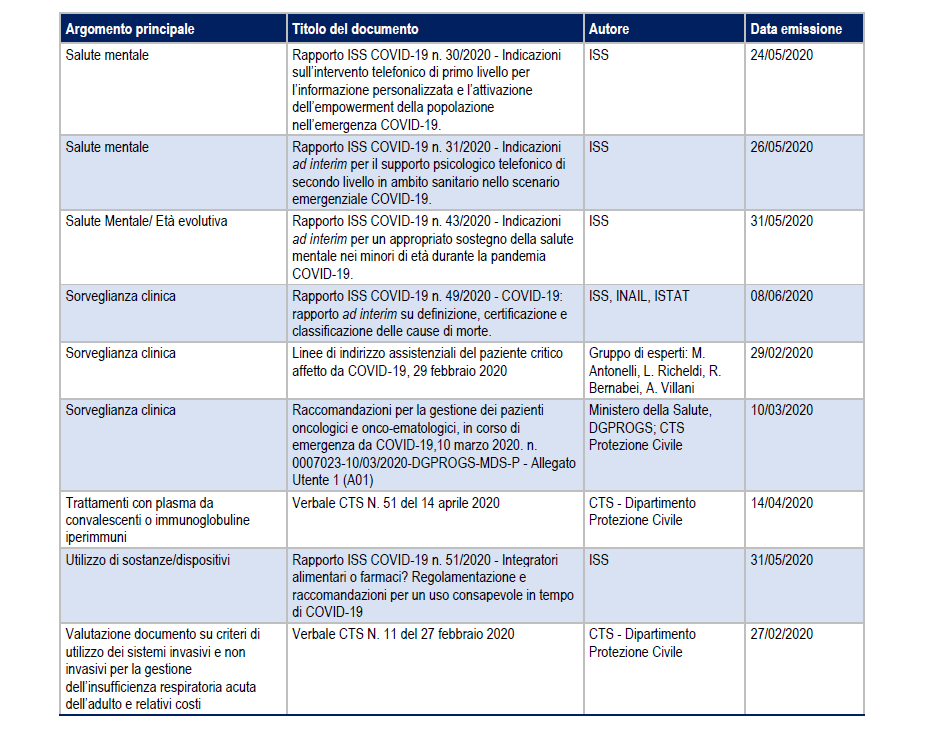
Here are the anti Covid drugs that work (and those that don’t)

Covid-19: recommended therapies, ongoing trials and non-recommended drugs. The report "Prevention and response to COVID-19: evolution of strategy and planning in the transition phase for the autumn-winter period", by the Ministry of Health and the Istituto Superiore di Sanità
How to cure Covid-19? What drugs are used against Sars-Cov-2?
Even today there is no official and dedicated drug, although the clinical management of patients has evolved over time.
Now the pandemic appears to be more manageable, but concern over the winter months remains.
Here is how Italy has treated and treated the Covid-19 disease, the evidence that has emerged in recent months and future plans, according to a report by the Ministry of Health.
NO DEDICATED DRUGS
Let's start with a fact: we do not have a certain and dedicated cure against Covid and there are still many uncertainties about the evidence.
"It should be opportunely remembered that, even today, there are large margins of uncertainty regarding the effectiveness of some of the aforementioned therapeutic cornerstones and the use of different therapies rather than the absence of their use depend on the severity of the clinical manifestations presented by sick people. Not coincidentally, there is a strong recommendation that above all patients with the most severe symptoms (hospitalized patients) be included in clinical trials whose conduct is aimed at defining the role of the various treatment options in a conclusive manner ", reads the report" Prevention and response to COVID-19: evolution of strategy and planning in the transition phase for the autumn-winter period ”, signed by the Ministry of Health and the Istituto Superiore di Sanità .
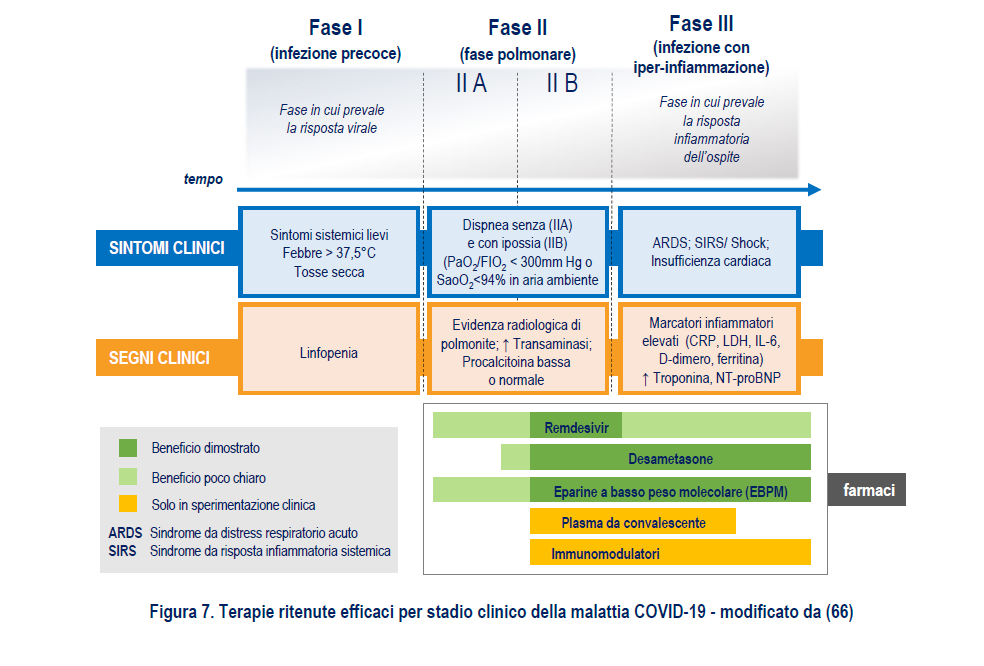
THE CLINICAL STAGES OF THE DISEASE
The medical approach and therapies were chosen based on the Sars-Cov-2 classification: asymptomatic infection, mild disease, moderate disease, severe disease and critical illness.
EFFECTIVE THERAPIES (AND THOSE NOT)
Among the drugs considered effective are Remdesivir, Dexamethasone and low molecular weight heparins. In clinical trials there is convalescent plasma and immunomodulator therapy.
THE CORTICOSTEROIDS
Recommended for the treatment of Covid-19 are corticosteroids, recommended by the main international guidelines for the care of hospitalized patients with severe COVID-19 disease who need oxygen supplementation (including those on invasive and non-invasive mechanical ventilation) .
"Glucocorticoids represent the only class of drugs that have shown a benefit in terms of reducing mortality", reads the report, which specifies that "the main evidence supporting the use of dexamethasone in Covid-19 derives from the study RECOVERY (Randomized Evaluation of Covid-19 Therapy), an open-label randomized controlled study conducted in the UK under the aegis of the Randomized Evaluation of Covid-19 Therapy, which compared different treatments in patients hospitalized with Covid-19. Analysis of 6,425 randomized subjects (2,104 in the dexamethasone arm and 4,321 in the usual care arm) showed statistically lower mortality in the dexamethasone arm than in the control arm in the general population (22.9% vs 25, 7%; RR 0.83; 95% CI 0.75-0.93; p <0.001) ".
REMDESIVIR
The use of remdesivir, a drug developed by the American Gilead , is recommended "in hospitalized patients with severe Covid-19 disease, who require standard oxygen supplementation, but who do not require high flow oxygen and mechanical ventilation", specifies the report. The total duration of the treatment must be at least 5 days and must not exceed 10 days.
The efficacy of the treatment was mainly evidenced by the ACTT-1 study, a randomized, double-blind, multinational clinical trial sponsored by the National Health Institutes.
"The data obtained showed, in the general population of hospitalized patients with Covid-19, a statistically significant 4-day superiority of remdesivir compared to placebo in the clinical recovery time in patients in the remdesivir group compared to those in the placebo group (11 vs 15 HR days: 1.32; 95% CI 1.12-1.55; p <0.001). In the population stratum presenting pneumonia and need for supplemental oxygen, the difference in median recovery time was 12 days in the remdesivir group versus 18 in the placebo group (RR 1.36; 95% CI 1.143-1.623; p <0.001) " , specifies the report, which adds: “In the general population receiving remdesivir a more favorable trend in terms of mortality was observed at 14 compared to the placebo group without reaching statistical significance; HR 0.70; 95% CI 0.47-1.04) ".
LOW MOLECULAR WEIGHT HEPARINS
The use of low molecular weight heparins is recommended "in the prophylaxis of thrombo-embolic events in patients with acute respiratory infection and reduced mobility", who have "levels of D-dimer much higher than normal (4-6 times) and / or a high score on a "sepsis induced coagulopathy" scale.
A retrospective analysis of 415 consecutive cases of severe ongoing Covid-19 pneumonia admitted to the Chinese hospital in Wuhan showed that "the administration of heparin (unfractionated or LMWH) for at least 7 days could lead to an advantage in terms of survival ".
IMMUNOMODULATORS
The immunomodulators, monoclonal antibodies anti-IL-6, anti-IL-1, tyrosine kinase inhibitors do not seem to have demonstrated efficacy.
There is still no evidence of their effectiveness. "The role of immunomodulators remains controversial in the literature," the report reads.
"Preliminary reports of two studies related respectively to the use of Tocilizumab and Sarilumab seem to indicate a lack of treatment benefit in the populations studied", says the report, which also states that "data from the phase III study have recently been disclosed EMPACTA which would demonstrate an advantage of Tocilizumab over the standard of care in terms of progression to mechanical ventilation or death ”.
CONVALESCENT PLASMA
Poor efficacy even for convalescent plasma therapies, literature data available at the moment do not allow to support recommendations regarding the use of the product.
"A clinical trial conducted in China in the period February-April 2020, but terminated early due to the difficulties of enrollment due to the epidemiological evolution of the epidemic, in which 103 subjects with severe or critical stage COVID were enrolled, demonstrated a benefit of convalescent plasma over standard of care in terms of time to clinical improvement in the subgroup of subjects with severe disease. On the contrary, there was no efficacy related to plasma infusion in subjects with less severe manifestations attributable to Covid-19 as well as in patients in a critical / advanced situation ".
MONOCLONAL ANTIBODIES: CLINICAL DEVELOPMENT
The use of monoclonal antibodies that could neutralize the virus and represent an important therapeutic option in Cpvid-19 patients is being tested. The therapy was administered, among others, to Donald Trump who is cheering on the FDA's authorization for their emergency use.
DRUGS NOT RECOMMENDED
Several drugs for which Aifa has suspended off-label use for chloroquine and hydroxychloroquine (29 May 2020), lopinavir / ritonavir and darunavir / cobicistat (17 July 2020), whose use is currently only foreseen within clinical trials. chloroquine and hydroxychloroquine (29 May 2020), lopinavir / ritonavir and darunavir / cobicistat (17 July 2020), whose use is currently only foreseen in clinical trials ".
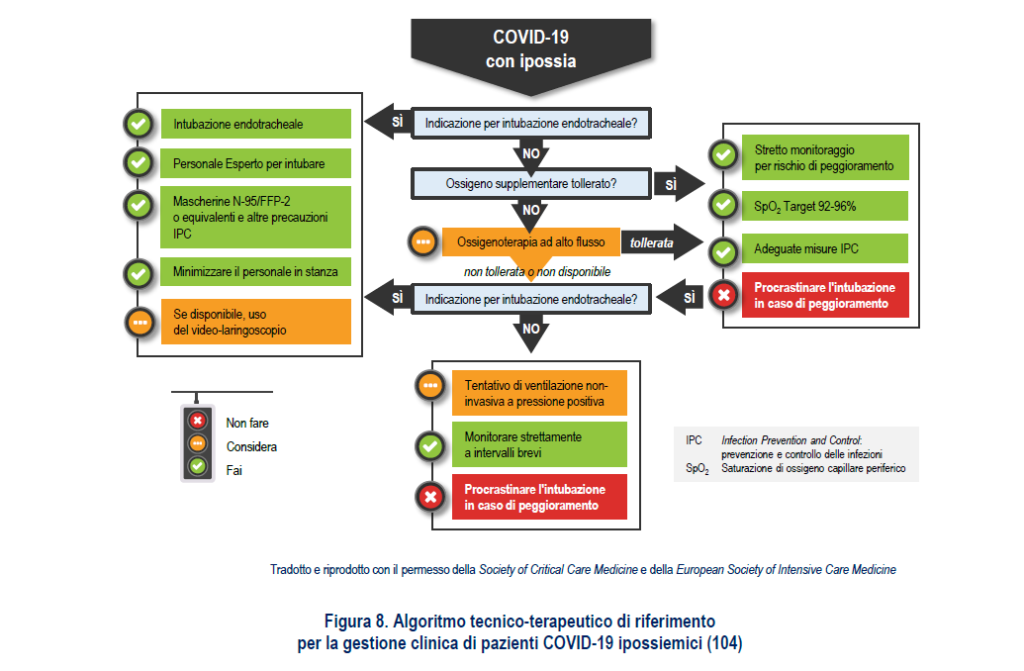
OXYGEN THERAPY AND PRONE POSITION
And again, the report of the Ministry of Health and the Istituto Superiore di Sanità specifies: "Like all the ARDS treated so far, even severe respiratory insufficiency from Covid-19 has seen the application of traditional oxygen therapy techniques to high humidified flows and heated (HFOT), non-invasive and invasive protective ventilation (low current volumes, moderate levels of end-expiratory pressure (PEEP) calibrated according to the respiratory response, as well as the use of drugs with neuromuscular blocking action in the first 24- 48 hours, of pronation techniques up to the use of ECMO extracorporeal oxygenation) (103). In compliance with the recommendations, the use of the various techniques was modulated, as usual, according to the severity of the respiratory situation ".
BEDS AND FANS
Alongside the use and experimentation of drugs, Italy has responded to the pandemic from Covid-19 by increasing the number of beds in intensive care: "an increase of up to 8679 PL has been established, resulting in an expansion of IT from 12 to 14 per 100,000 inhabitants, thus meeting the standards recommended by international companies in the sector ”, reads the report.
The supply of mechanical fans has also increased: “The commissioner structure delegated to combat the emergency from COVID-19 has taken charge of recovering the fans necessary for the emergency, now part of the consolidated armory of IT. In the month of March alone, the commissioner structure delivered 1,231 ventilators and 6,831 helmets for CPAP ”.
HOW THE GOVERNMENT PREPARES TO FACE THE PANDEMIC FOR THE AUTUMN-WINTER 2020-2021
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/innovazione/ecco-i-farmaci-anti-covid-che-funzionano-e-quelli-che-non-funzionano/ on Tue, 13 Oct 2020 12:15:42 +0000.
