Vaccines, adverse reactions, deaths. Aifa report

What is the most used vaccine? And what causes the most adverse reactions? Are more men or women reporting suspected cases? This and much more (including administration-related deaths) in the Aifa report
The ninth Pharmacovigilance Report on Covid vaccines published by the Italian Medicines Agency (Aifa) shows the data collected and recorded by the National Pharmacovigilance Network between 27 December 2020 and 26 September 2021.
In particular, it focuses on reports of suspected adverse reactions following the administration of the four vaccines in use in the current vaccination campaign (Pfizer / BioNTech, Moderna, AstraZeneca, Johnson & Johnson).
DATA ON REPORTS
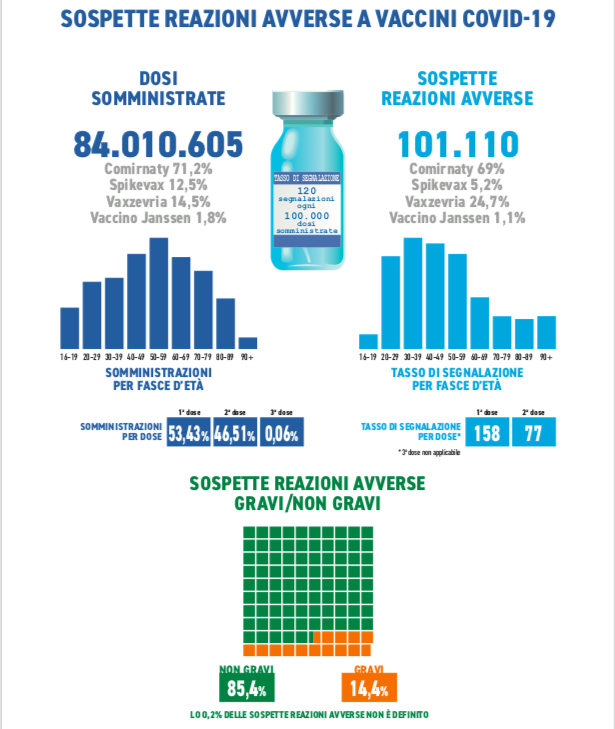
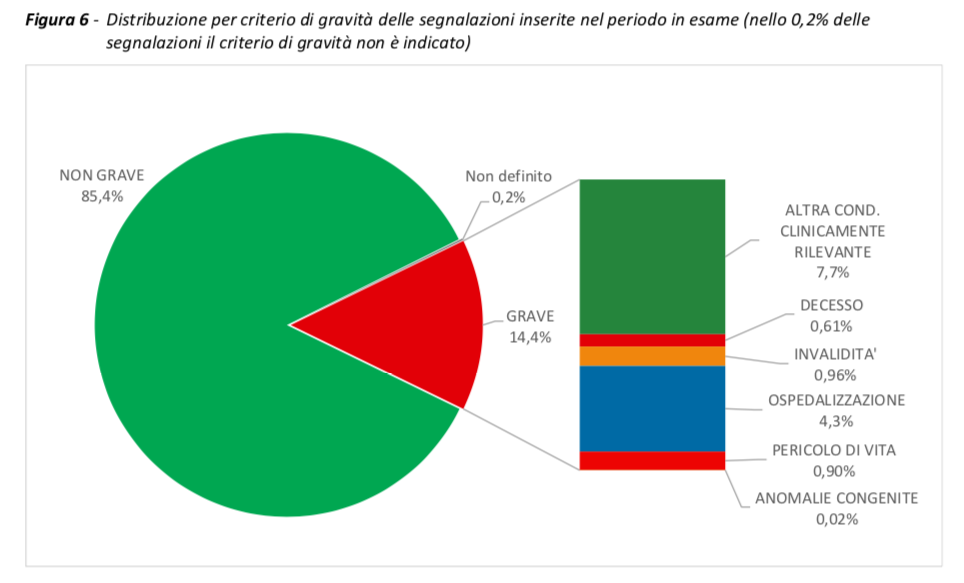
In the period considered, 101,110 reports were received out of a total of 84,010,605 doses administered (reporting rate of 120 per 100,000 doses), of which 85.4% referred to non-serious events, such as pain at the injection site, fever , asthenia / fatigue, muscle aches.
Serious reports correspond to 14.4% of the total, with a rate of 17 serious events per 100,000 doses administered.
As reported in previous Reports, regardless of the vaccine, dose and type of event, the reaction occurred in most cases (about 76%) on the same day of vaccination or the next day and only more rarely beyond 48 hours. .
REPORTS BY AGE, GENDER AND TYPE OF REPORTER
The mean age of people who have experienced a suspected adverse event is 47.8 years (median age of 48 years).
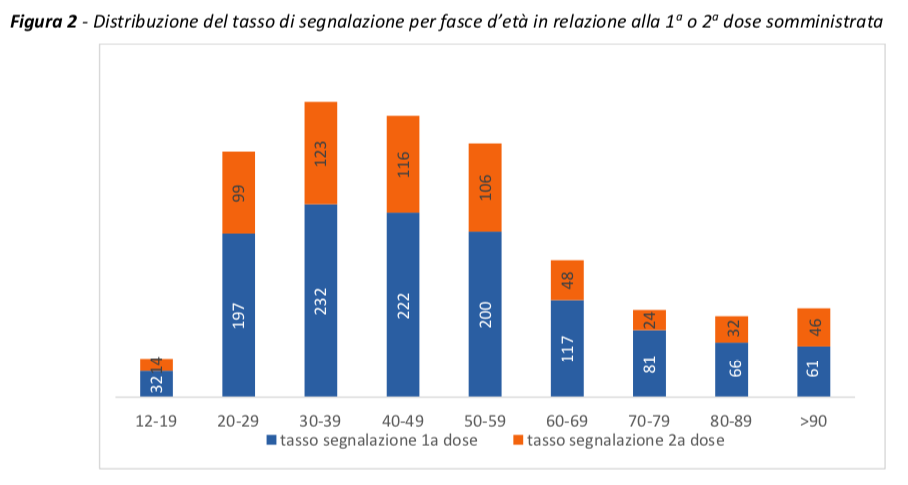
As already reported in the pre-authorization clinical studies and in the previous Reports, the reporting rate is higher in the age groups between 20 and 60 years, and then decreases in the more advanced age groups and in the very young, with a rate of lower signaling after the second dose.
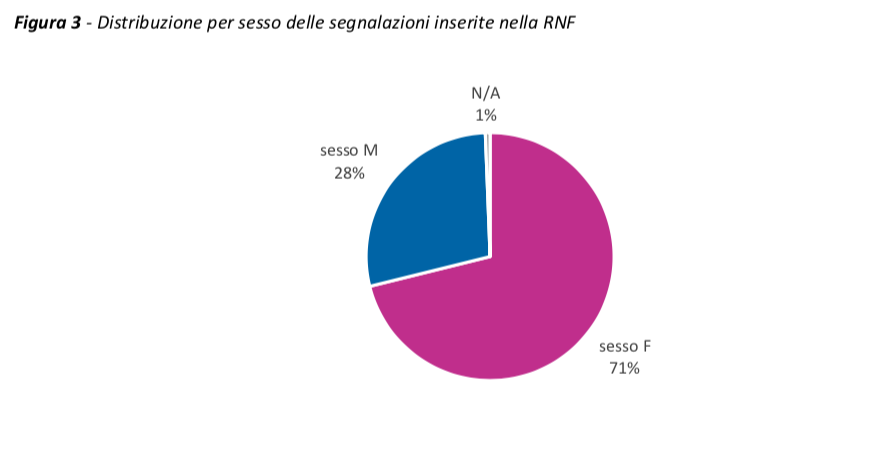
Compared to a comparable exposure between the sexes (52% of the doses administered in females and 48% in males), 71% of the reports concern women (166 / 100,000 doses administered) and 28% men (70 / 100,000 doses administered), regardless of the vaccine and dose administered.
The same trend can also be observed in the other European countries.
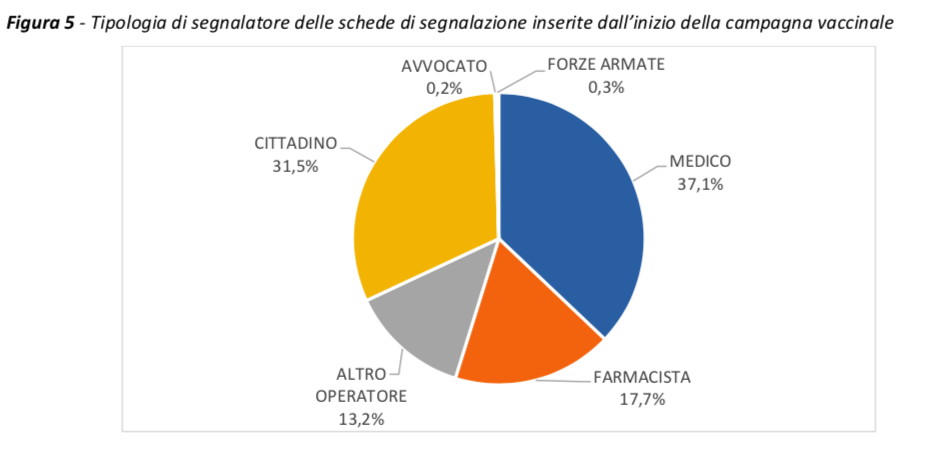
About 68% of the reports come from health professionals, mainly doctors and pharmacists, while about 31.5% from patients / citizens, with a modest increase compared to previous months.
WHAT IS THE MOST USED VACCINE?
Comirnaty, Pfizer's vaccine, is currently most used in the Italian vaccination campaign (71.2%); followed by that of AstraZeneca, Vaxzevria (14.5%); Spikevax by Moderna (12.5%) and finally the single-dose by J&J, Janssen (1.8%).
FOR WHICH VACCINE WERE THE MOST REPORTED?
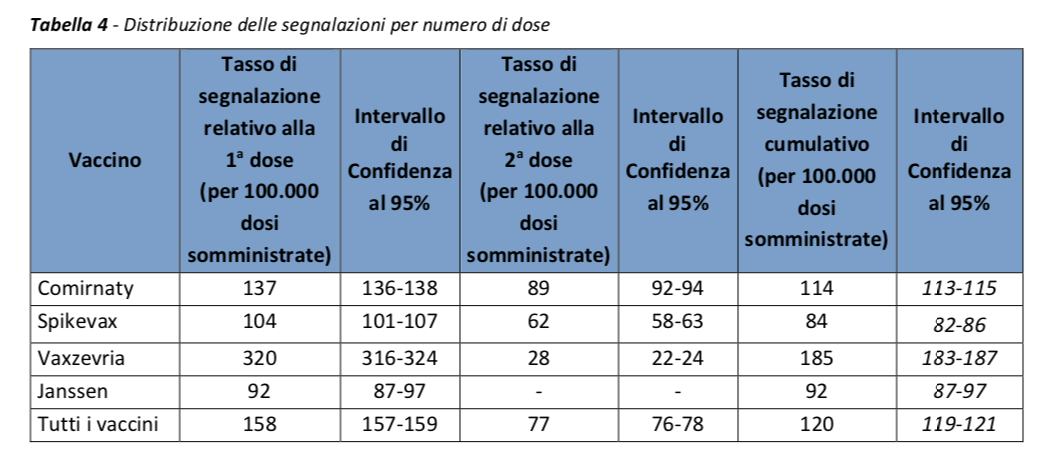
In line with previous Reports, the distribution of reports by type of vaccine is similar to that of administrations (Comirnaty 68%, Vaxzevria 22%, Spikevax 9%, Janssen 1%).
As noted in previous Reports, the reporting rates for the second dose are lower than those for the 1st dose, with a very pronounced difference for the AstraZeneca vaccine.
For all vaccines, the most reported adverse events are fever, fatigue, headache, muscle / joint pain, local reaction or pain at the injection site, chills and nausea.
HOW MANY REPORTS ARE CONSIDERED SERIOUS?
Following the Pfizer vaccine, approximately 4 reports per 100,000 doses administered have been classified as serious. For every million doses administered there were 6 cases of myocarditis / pericarditis, 3 cases of anaphylactic reactions and 2 cases of facial paralysis.
The serious reports related to vaccination in the case of Moderna are about 3 for every 100,000 doses administered. For every million doses administered, the number of cases of myocarditis / pericarditis that reaches 11 is slightly higher than the Pfizer vaccine, while 2 cases of anaphylactic reactions and 2 cases of facial paralysis are reported.
For every 100,000 administered doses of AstraZeneca vaccine 11 reports were considered serious correlatable. Some adverse events continue to be very rare: for every million doses administered there are 2 cases of anaphylactic reaction and around 1 case for acute and subacute neuropathies (including Guillain-Barrè syndrome), intracranial or on-site venous thrombosis atypical with thrombocytopenia (VITT) and idiopathic thrombocytopenia.
After the administration of J&J there are about 5 reports per 100,000 doses found to be serious related to vaccination. For every million doses administered, the number of cases of cerebral or atypical site venous thrombosis with thrombocytopenia, acute or subacute polyneuropathies, or severe allergic-type reactions have a national reporting rate of less than 1.
DATA ON HETEROLOGICAL VACCINATIONS
In relation to the so-called heterologous vaccinations to people under 60 who had received Vaxzevria as the first dose, 262 reports were received, out of a total of 644,428 administrations (the second dose concerned Comirnaty in 76% of cases and Spikevax in 24%) , with a reporting rate of 40 per 100,000 doses administered.
SIGNALS IN THE 12-19 YEARS BAND
In the age group between 12 and 19 years, as of 09/26/2021, 1,358 reports of suspected adverse event were received out of a total of 5,623,932 doses administered, with a reporting rate of 24 adverse events per 100,000 doses administered.
The distribution by type of adverse events is not substantially different from that observed for all other age groups. Rare cardiological events are being investigated.
THIRD DOSE AND REPORTING
With regard to the administration of the third dose , which began in September, only one report was made, compared to approximately 46,000 doses administered.
GOOD NEWS
Given the stability of the reporting trend for the various anti Covid vaccines, the Surveillance Report will no longer be published monthly but quarterly. On the other hand, the interactive graphs available on the Aifa website remain monthly.
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/vaccini-reazioni-avverse-decessi-report-aifa/ on Wed, 13 Oct 2021 09:01:13 +0000.
