What (not) goes in heterologous vaccination. Gimbe Report

The analysis of the president of the Gimbe foundation, Nino Cartabellotta, on heterologous vaccination
«If immunological and biological assumptions and preliminary data suggest that“ heterologous ”vaccination is effective and safe, there remains the inconsistency between the obligation envisaged by the circular of the Ministry of Health and the possibility reported by the AIFA determination. In fact, according to Aifa's formula for under 60s, the second dose with Pfizer or Moderna is only an option that the patient is free to accept or refuse, opting for the second dose with AstraZeneca. In any case, it is essential to adapt the informed consent form to the provisions of Law 648/96 with adequate information on the benefits, risks and uncertainties of the options for the second dose after AstraZeneca ”.
This is the comment – with a sort of appeal to the Ministry of Health to clarify the question – of the president of the Gimbe foundation, Nino Cartabellotta, on heterologous vaccination, that is, on the second dose for those who made the first with the Astrazeneca vaccine.
"With regard to the new AstraZeneca chaos – adds Cartabellotta – if in the current context of low viral circulation the decision to limit this vaccine to the over 60s is totally acceptable, some perplexities emerge regarding the obligation to carry out the second dose with vaccine in the under 60s to mRNA, already renamed as “heterologous” ». In fact, in the under 60s who received the first dose of AstraZeneca, the circular of 11 June 2021 of the Ministry of Health states that "the cycle must be completed with a second dose of mRNA vaccine (Comirnaty or Moderna)".
Here are the points still to be clarified according to the Gimbe foundation:
- Scientific evidence: Despite the immunological, biological and some historical precedents on the vaccine mix, the scientific evidence is still preliminary. In particular, the 4 studies cited by the CTS opinion enroll just over 800 people and measure the effectiveness of the mix only on the immune response and safety only on frequent and short-term side effects. In other words, to date there is no evidence of efficacy of "heterologous" vaccination on severe Covid-19, hospitalizations and deaths, nor on any rare side effects.
- Regulatory aspects. At the date of publication of the circular of the Ministry of Health, the vaccine mix appeared to be off label, i.e. outside the authorized indications. The AIFA determination of 13 June 2021 "remedied" the problem, with reference to law 648/96 and by providing that mRNA vaccines "can be administered as a second dose to complete a mixed vaccination cycle". That is, the possible formula used by AIFA to allow the use of "heterologous" vaccination contrasts with the peremptory one provided for by the circular of the Ministry of Health.
- Informed consent and professional responsibility. The reference to Law 648/96 provides for the "informed written consent of the patient showing that the same is aware of the incompleteness of the data relating to the safety and efficacy of the medicinal product for the proposed therapeutic indication". That is, the law 648/96 requires the citizen to accept or not the information provided to him (if he does not sign the consent he cannot complete the vaccination cycle) and to the doctor the responsibility of the prescription, in the presence of an alternative whose efficacy profile and safety was reiterated by the EMA.
Here is the full report of the Gimbe foundation on the progress of the pandemic and the vaccination campaign in Italy.
+++
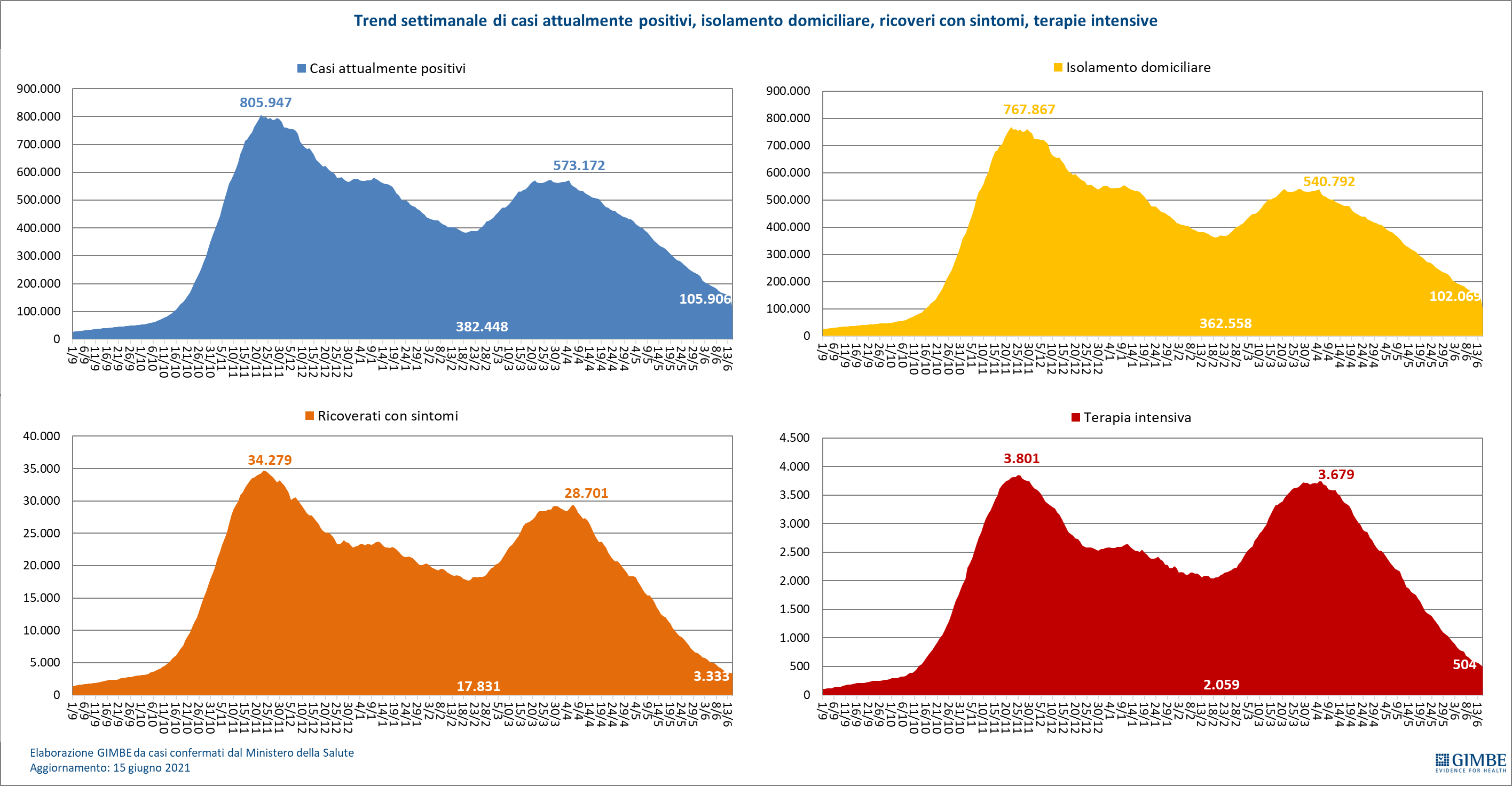
The independent monitoring of the Gimbe Foundation detects in the week 9-15 June 2021, compared to the previous one, a decrease in new cases (11,440 vs 15,288) (figure 1) and deaths (411 vs 469) (figure 2). Cases currently positive are also decreasing (105,906 vs 181,726), people in home isolation (102,069 vs 176,353), hospitalizations with symptoms (3,333 vs 4,685) and intensive care (504 vs 688) (figure 3). In detail, compared to the previous week, the following changes were recorded:
- Deaths: 411 (-12.4%)
- Intensive care: -184 (-26.7%)
- Hospitalized with symptoms: -1,352 (-28.9%)
- Home isolation: -74,284 (-42.1%)
- New cases: 11,440 (-25.2%)
- Currently positive cases: -75,820 (-41.7%)
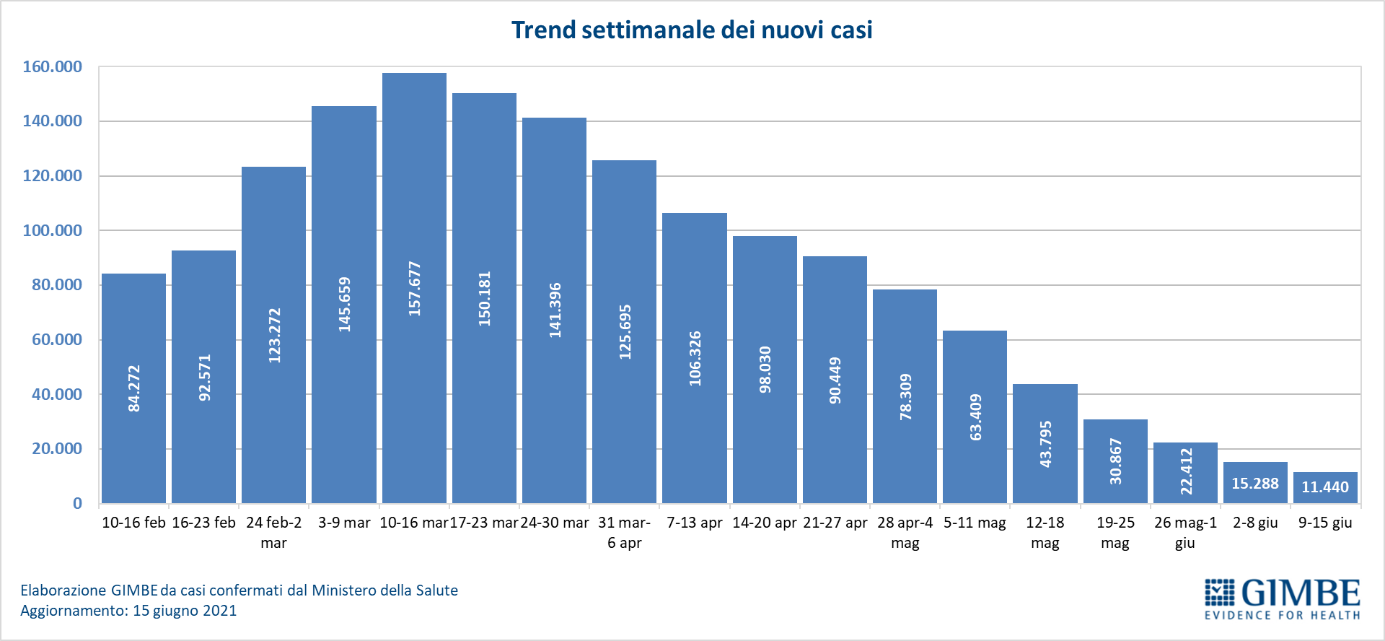
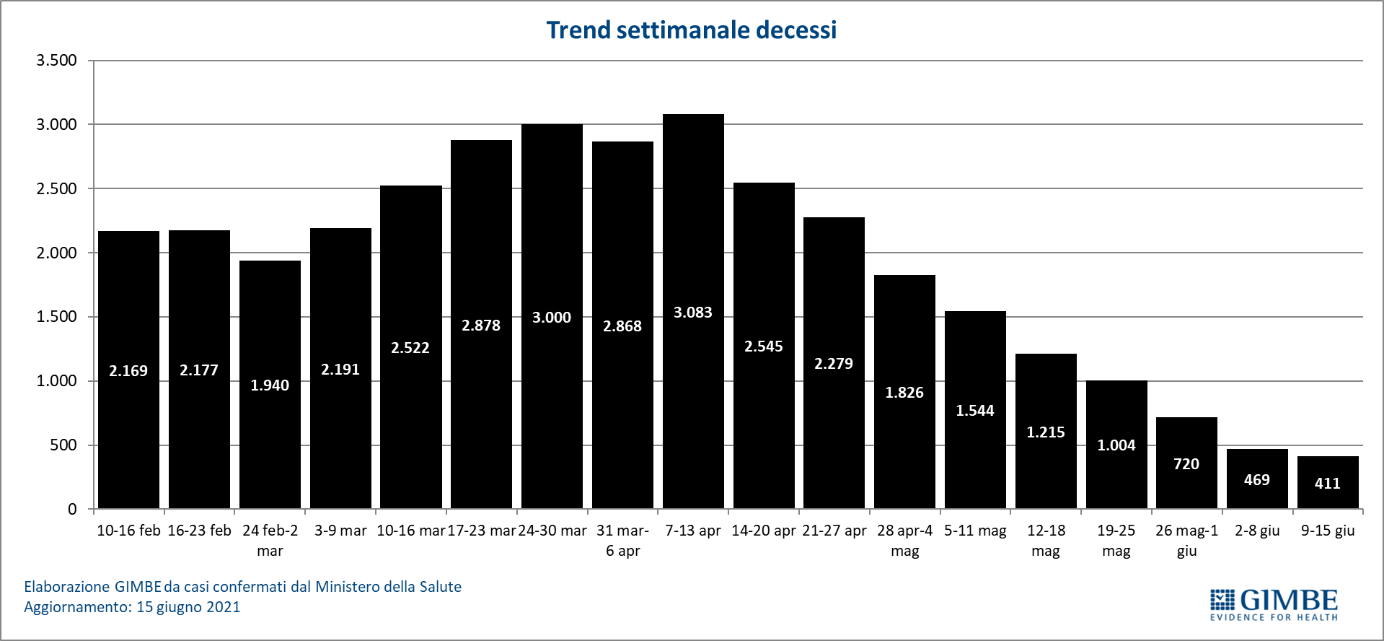
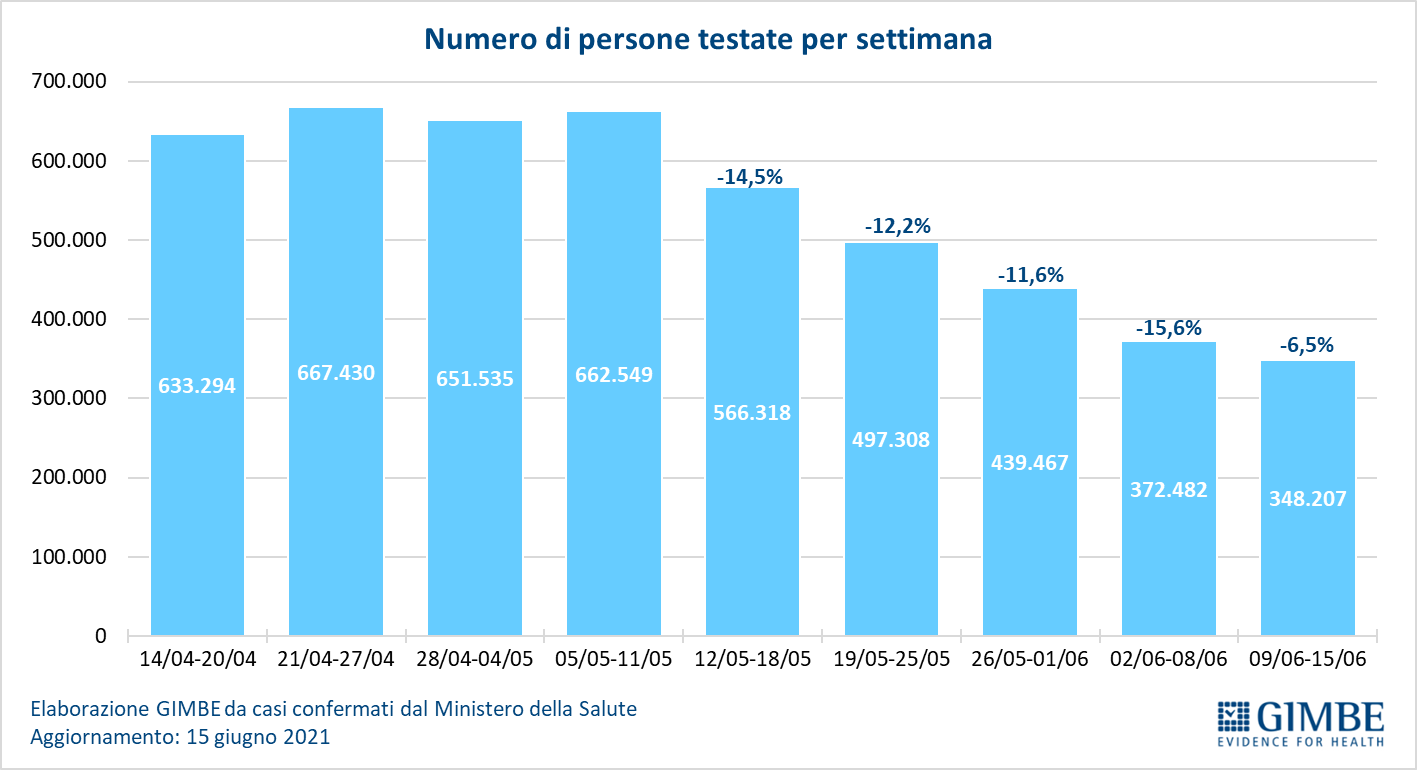
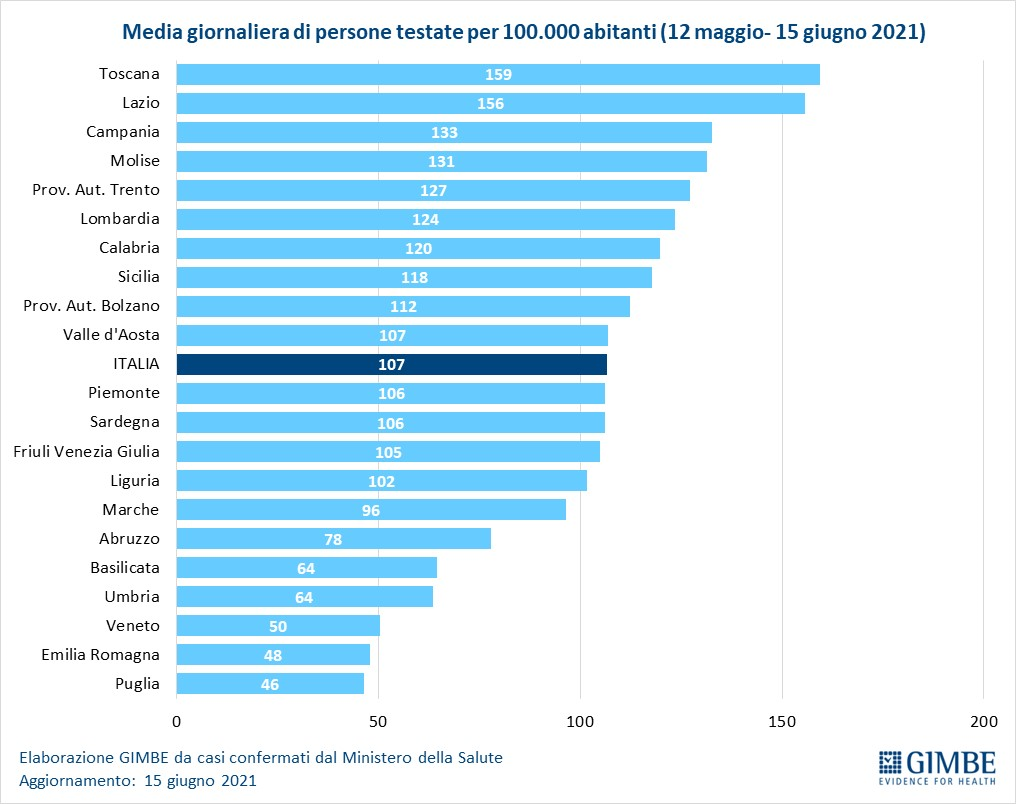
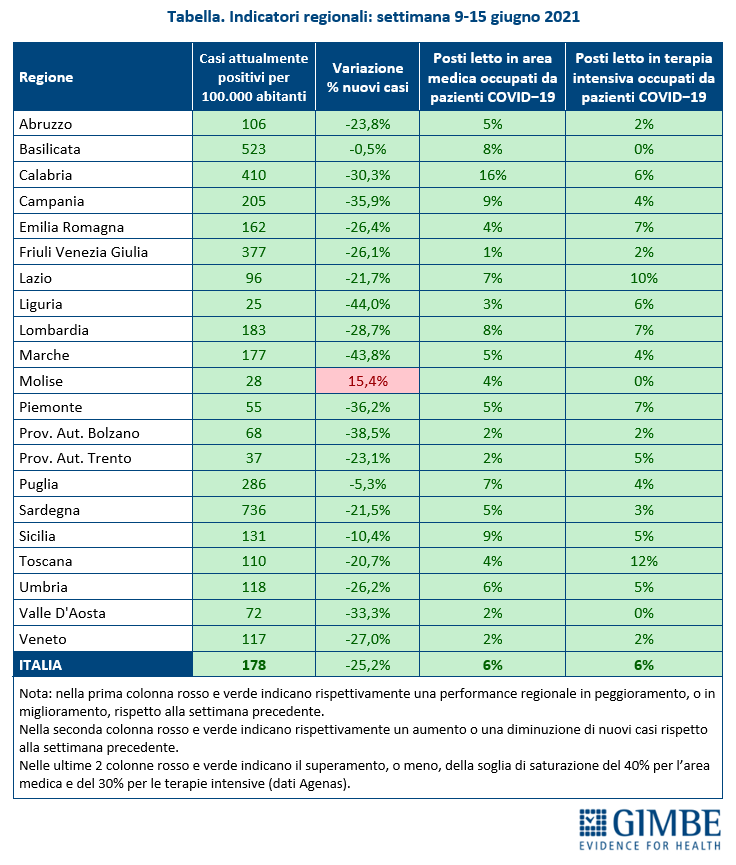
«For 13 consecutive weeks – declares Nino Cartabellotta, President of the Gimbe Foundation – there has been a decline in new weekly cases. If the constant reduction of the positive / tested cases ratio attests to a reduced circulation of the virus, the progressive decrease of the testing activity underestimates the number of new cases and documents the failure to resume contact tracing, which is fundamental in this phase of the pandemic ". In fact, in the last 5 weeks, the number of people tested fell by 31.5%, dropping from 3,247,816 to 2,223,782 (figure 4), with a national average of 132 people tested / day per 100,000 inhabitants and relevant and unjustified regional differences (Figure 5). In all Regions the decrease in new weekly cases is confirmed (the percentage increase in Molise is irrelevant in absolute value) (table). In addition, deaths have also been steadily decreasing for 9 weeks, reaching an average of 59 per day in the last week.
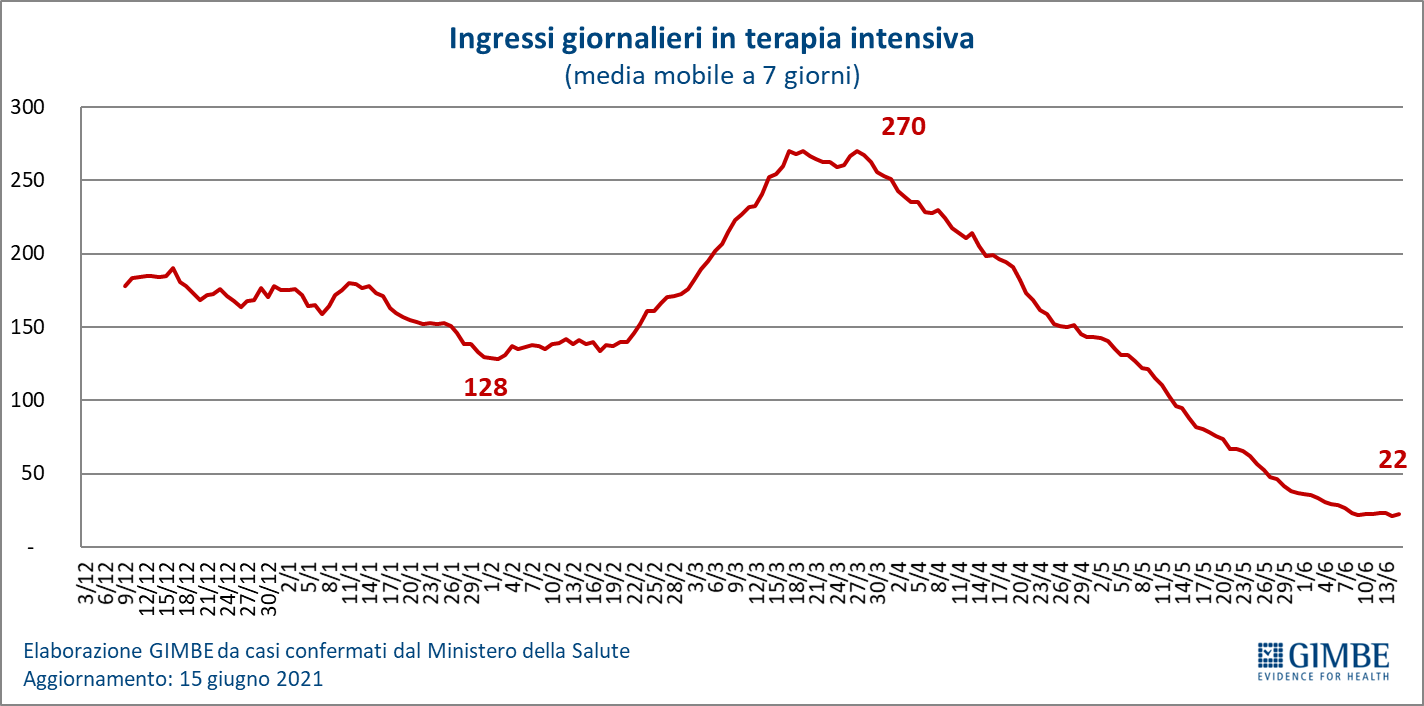
"The constant reduction of hospitalized patients – says Renata Gili, Head of Research on Health Services of the Gimbe Foundation – has brought the occupation of beds by Covid patients to 6% both in the medical area and in intensive care, with all Regions well below the alert thresholds ". In detail, from the peak of April 6, the number of beds occupied in the medical area fell from 29,337 to 3,333 (-88.6%) and those in intensive care from 3,743 to 504 (-86.5%). Following the adjustment of the Campania Region, then, from the peak of March 28, people in home isolation fell from 540,855 to 102,069 (-81.1%). "Daily admissions to intensive care – explains Marco Mosti, Operations Director of the Gimbe Foundation – have fallen for 10 consecutive weeks and are now stable with a 7-day moving average of 22 admissions / day" (figure 6).
Delta variant.
According to the latest variant prevalence survey published by the Istituto Superiore di Sanità on May 18, the delta variant (about 60% more contagious than the English variant) is 1% with regional differences and a range from 0 to 3.4%: in particular, the greatest diffusion is recorded in Lazio (3.4%), Sardinia (2.9%) and Lombardy (2.5%). However, in the last week the delta variant was isolated in two outbreaks in Milan and Brindisi, a sign of its greater diffusion on the national territory which is also detected by the international GISAID database: compared to sequencing on samples collected from 19 May to 16 June, out of 881 sequences deposited 57 (6.5%) correspond to the delta variant. Compared to the efficacy of vaccines, according to data from Public Health England, a single dose of vaccine (Pfizer-BioNTech or AstraZeneca) has an efficacy of only 33% against this variant, a percentage that rises after the second dose, respectively. 88% and 60%. Furthermore, the latest English study (Public Health England) certifies that the effectiveness of the complete cycle in preventing hospitalizations is 96% with the Pfizer-BioNTech vaccine and 92% with the AstraZeneca one.
Vaccines: supplies.
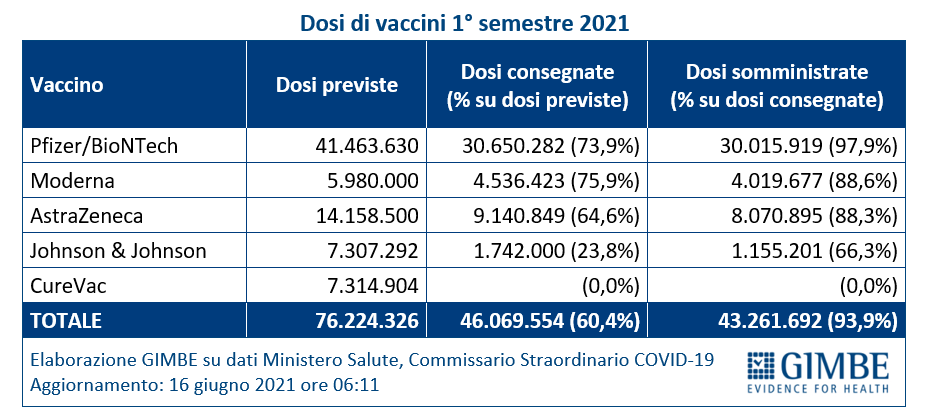
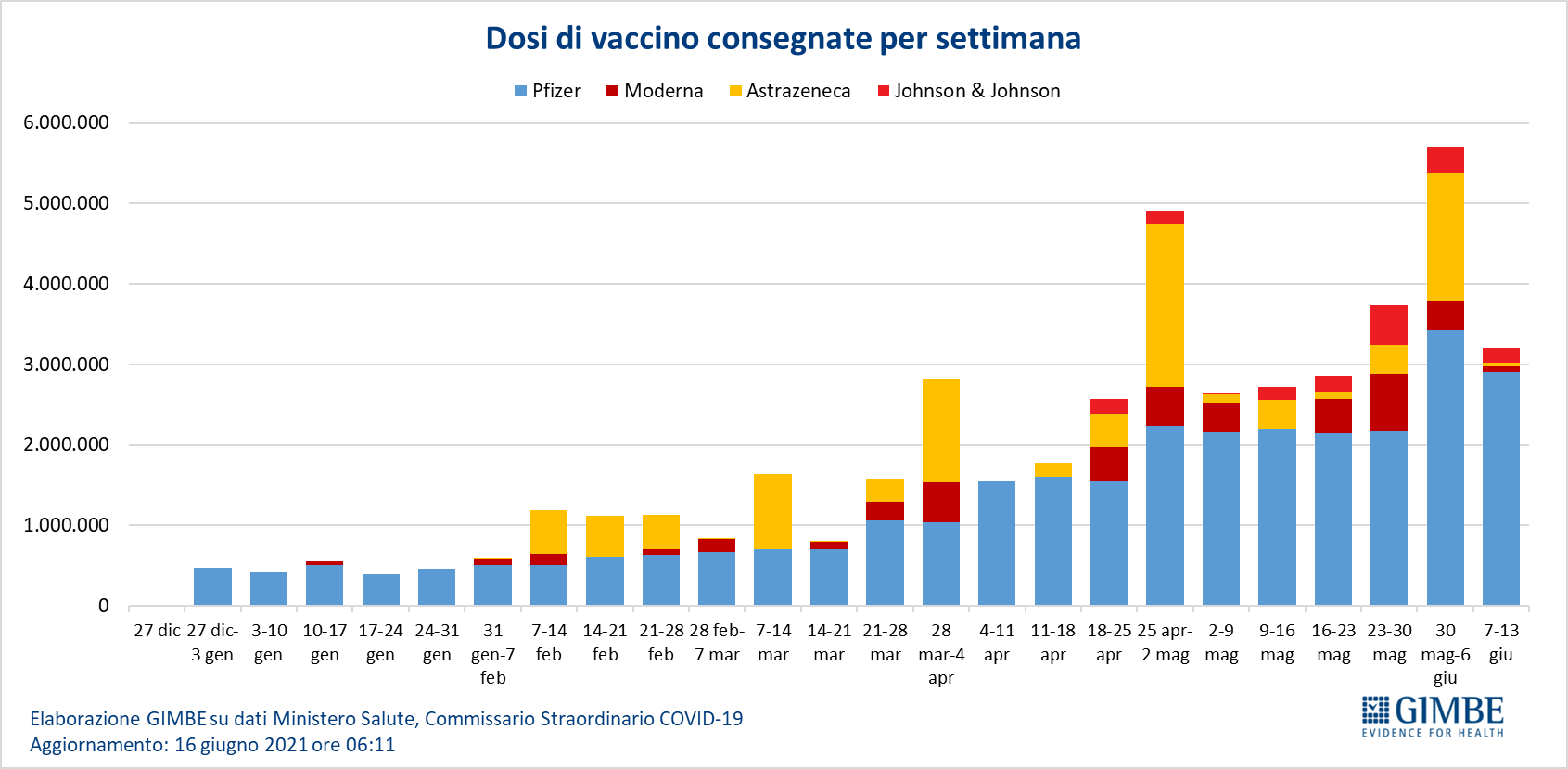
As of June 16 (update at 6.11) 46,069,554 doses were delivered, equal to 60.4% of those expected for the first half of 2021 (figure 7). In detail:
Vaccines: administrations.
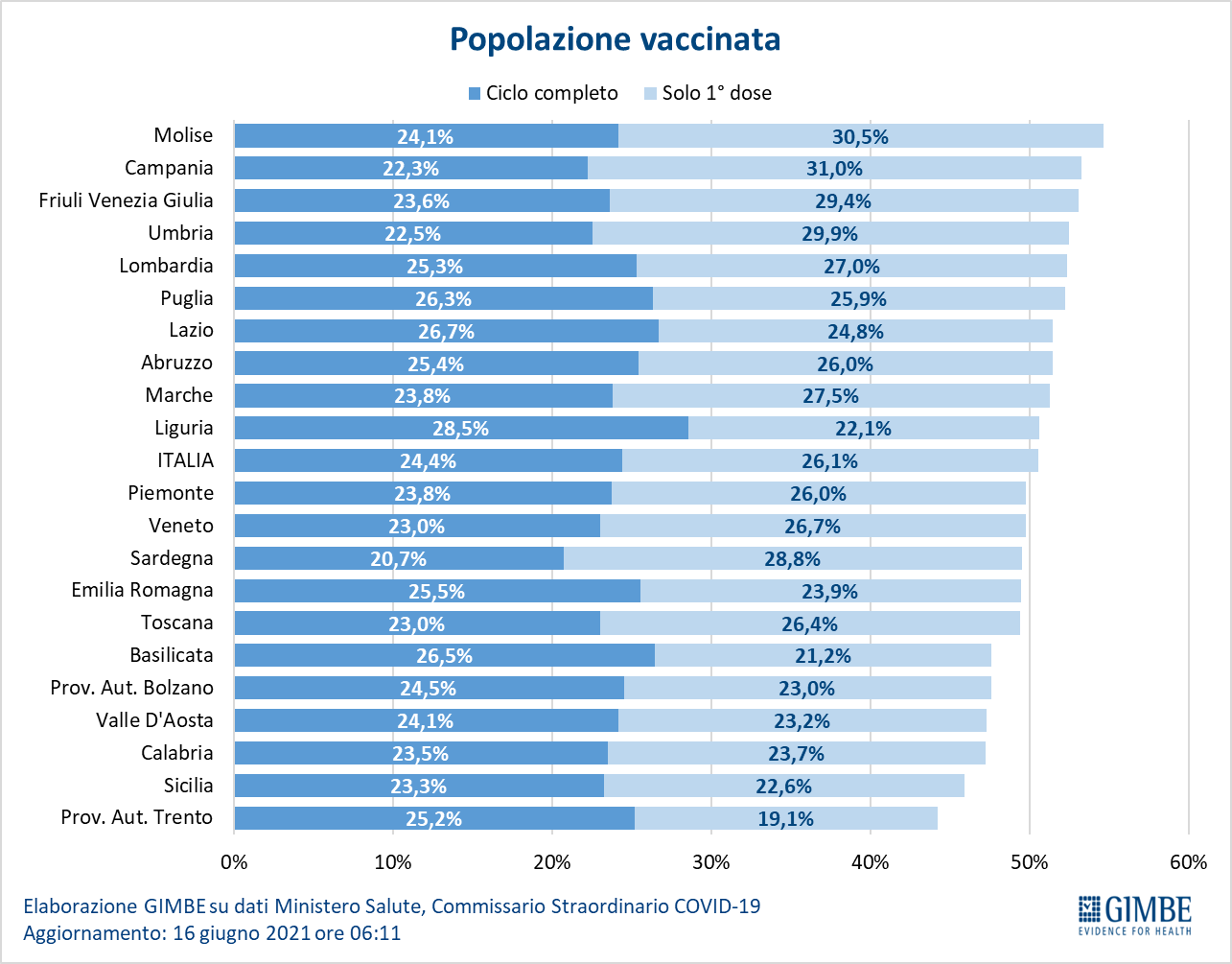
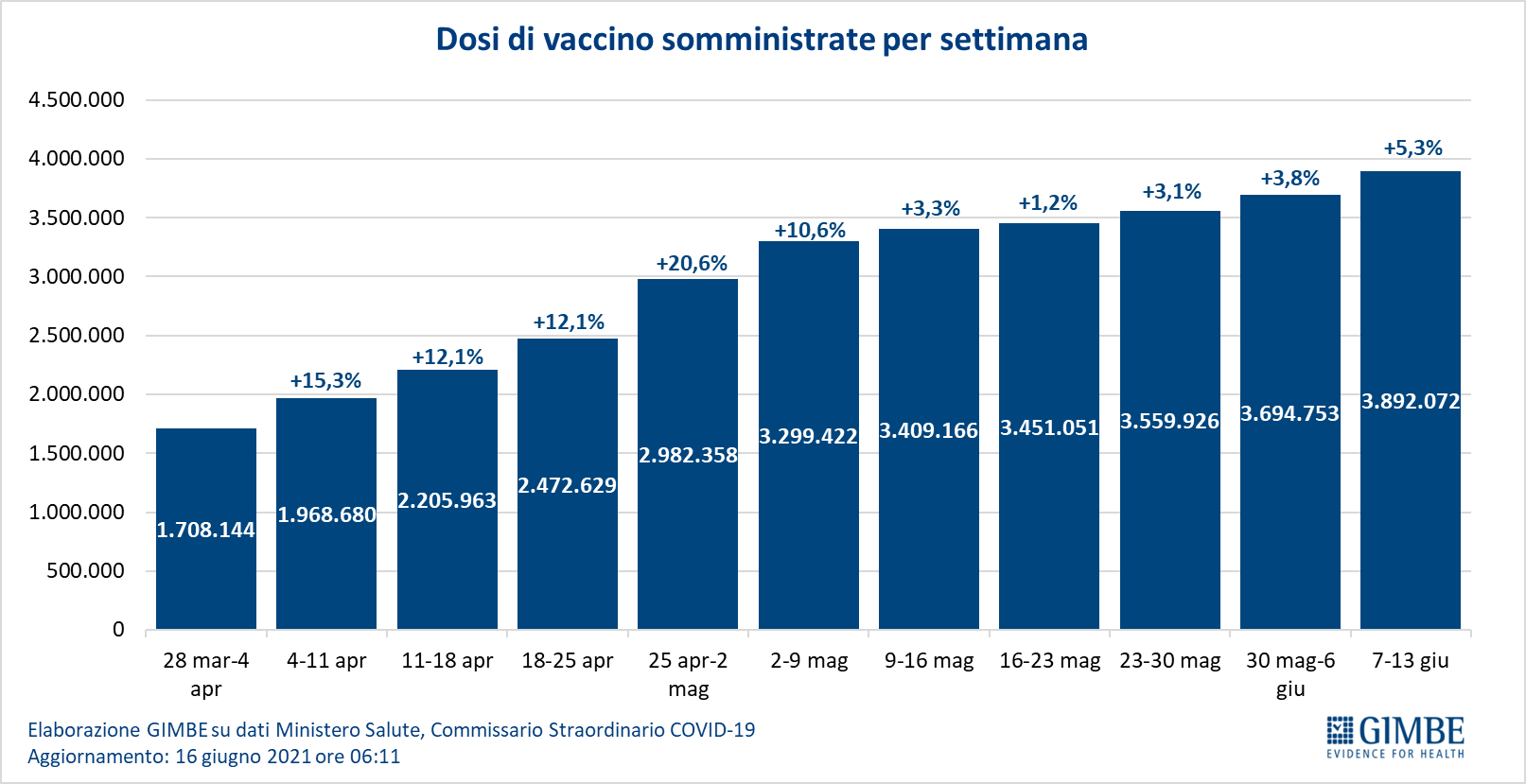
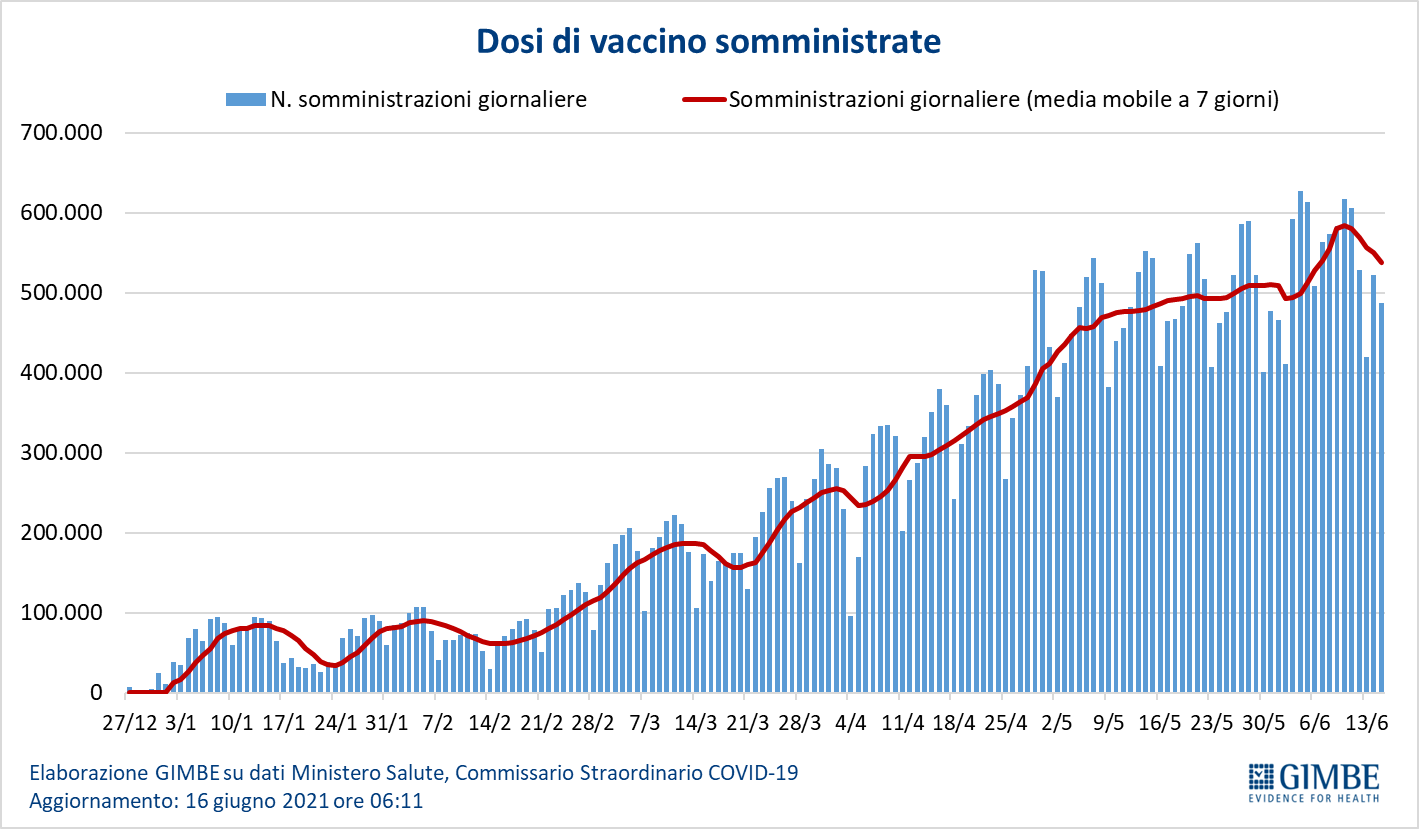
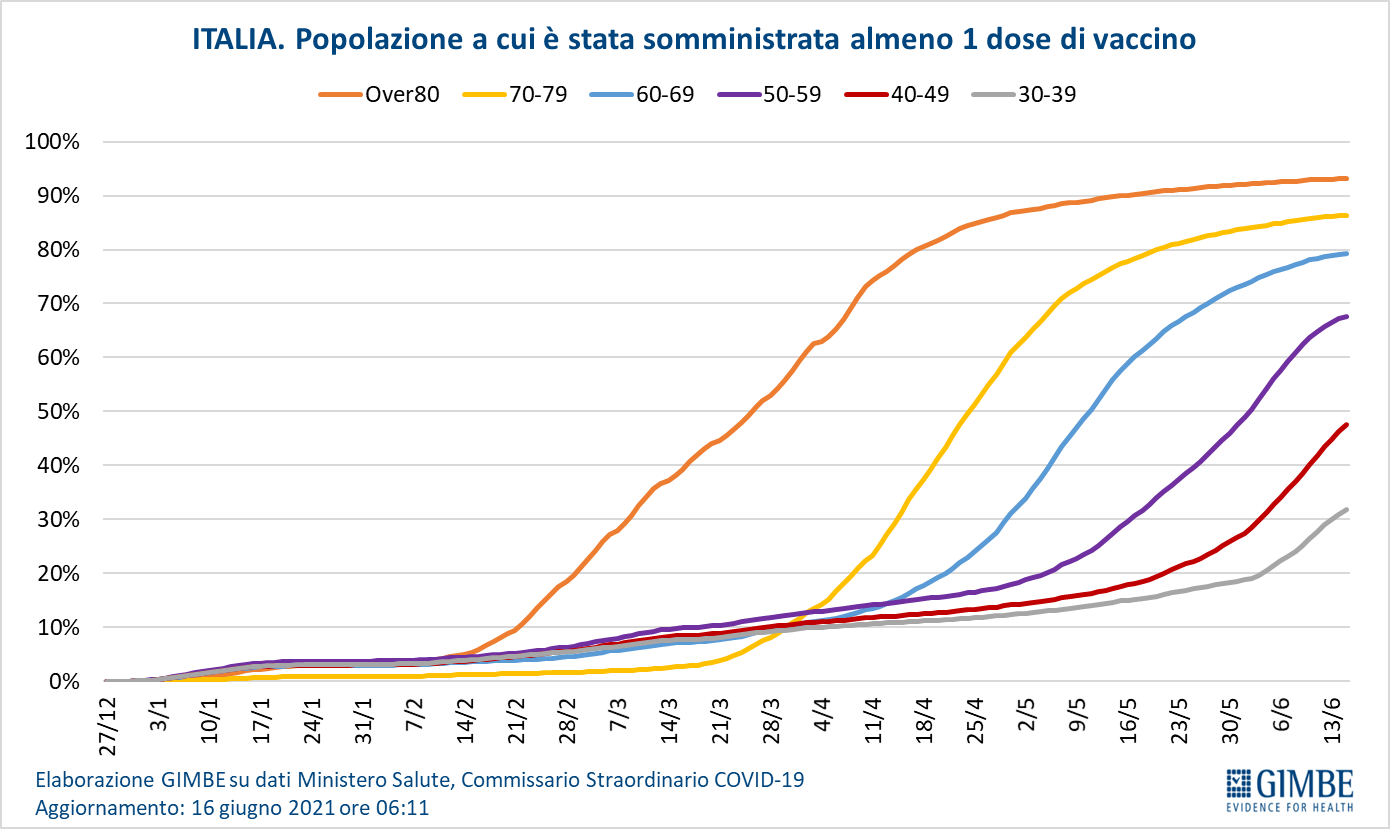
As of June 16 (updated at 6.11), 50.5% of the population received at least one dose of vaccine (n. 29,949,601) and 24.4% completed the vaccination cycle (n. 14,467,292) (figure 8). In the last week, 3,892,072 million injections were achieved (figure 9), with a 7-day moving average of 537,765,000 inoculations / day (figure 10).
Vaccines: coverage of priority categories.
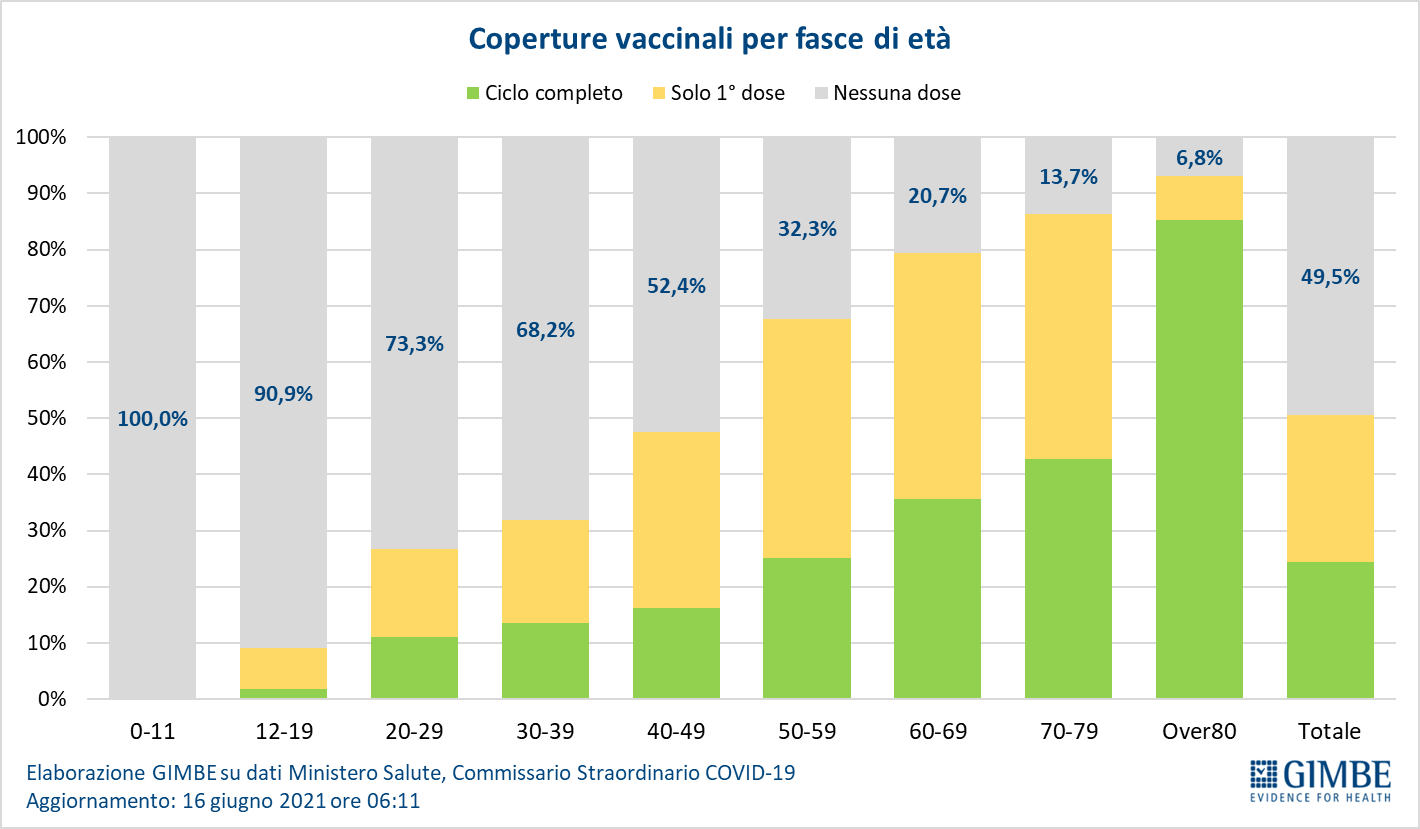
85.2% of the over 60s received at least the first dose of the vaccine, with some regional differences: if Puglia exceeds 90%, Sicily is under 75%. In detail:
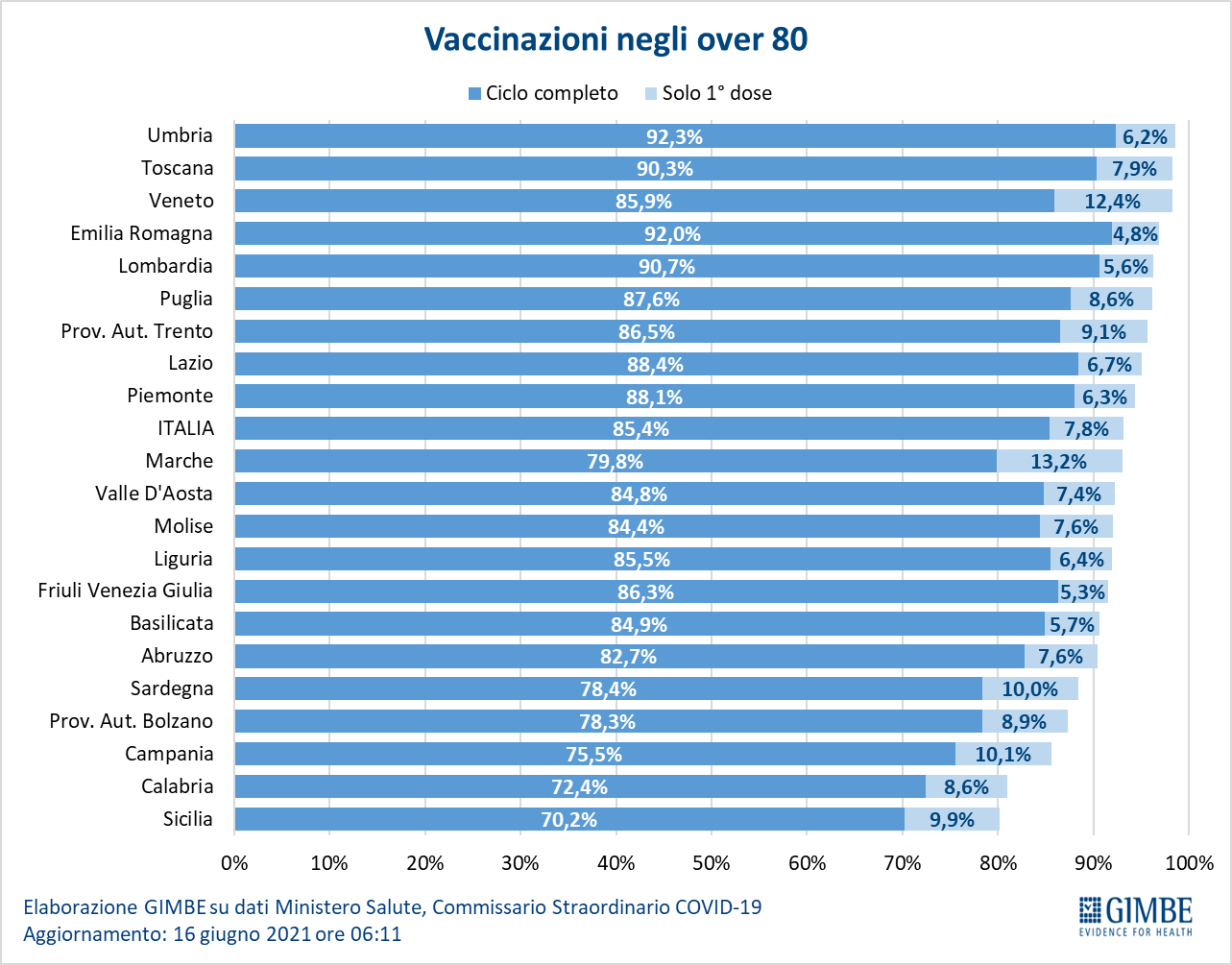
- Over 80: of the over 4.4 million, 3,824,604 (85.4%) completed the vaccination course and 349,498 (7.8%) received the first dose only (Figure 11).
- Age range 70-79: of the over 5.9 million, 2,544,393 (42.7%) completed the vaccination course and 2,605,613 (43.7%) received the first dose only (Figure 12).
- Age range 60-69: of the over 7.3 million, 2,655,476 (35.7%) completed the vaccination course and 3,247,643 (43.6%) received the first dose only (Figure 13).
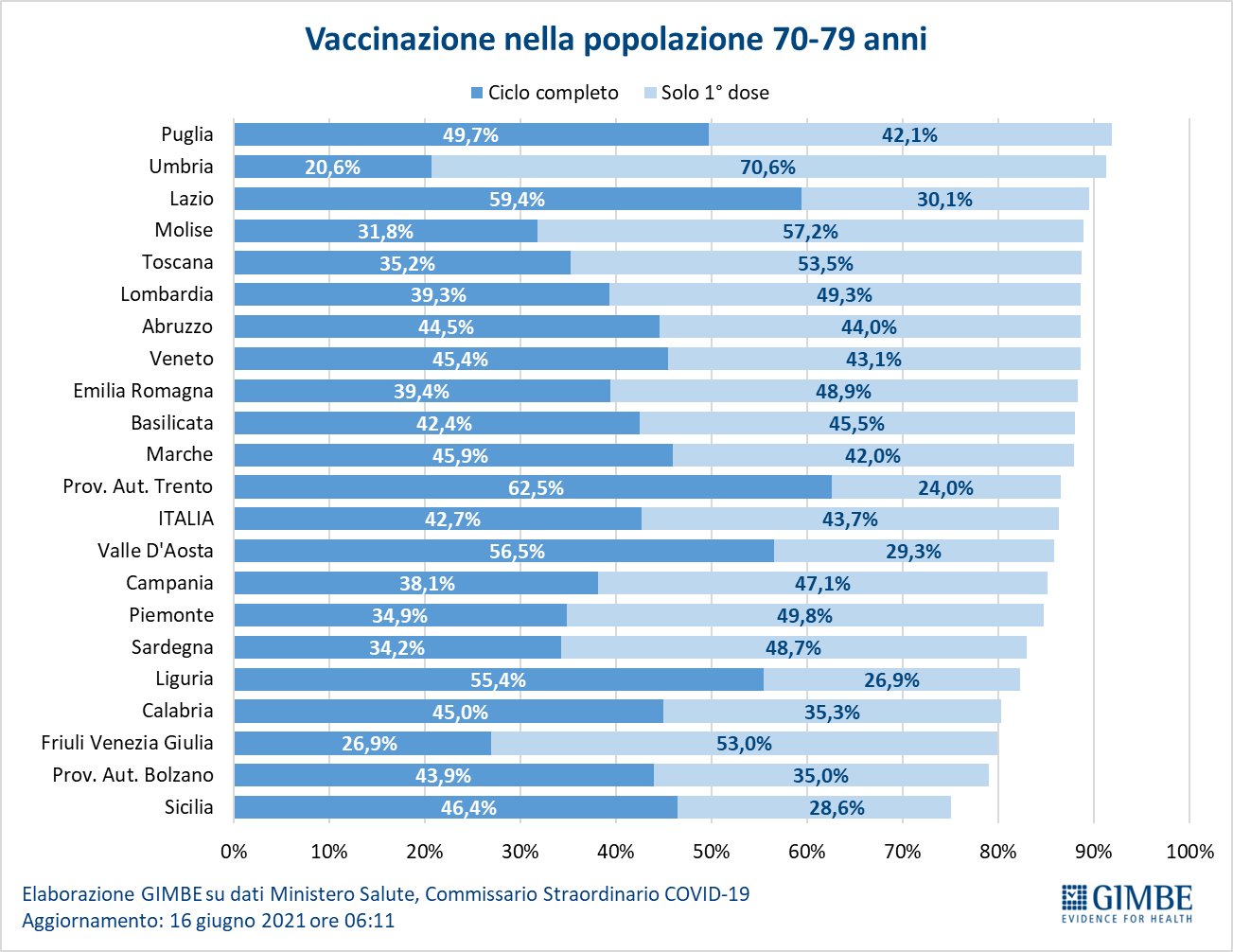
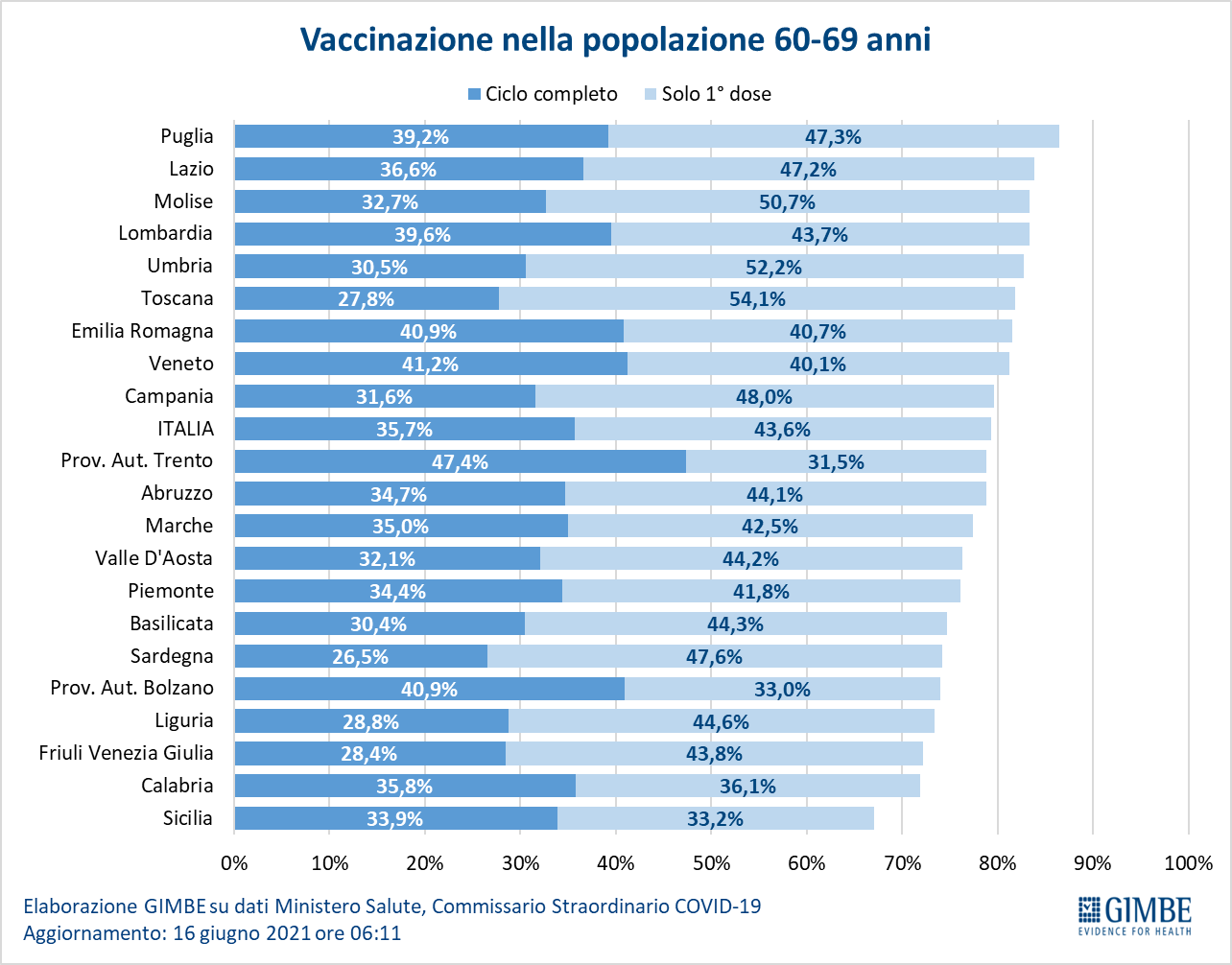
In the population over the age of 60, therefore, as many as 2.66 million have not yet received even the first dose of vaccine and 6.2 million have to complete the vaccination cycle (Figure 14).
"Heterologous" vaccination.
"With regard to the new AstraZeneca chaos – says Cartabellotta – if in the current context of low viral circulation the decision to limit this vaccine to the over 60s is totally acceptable, some perplexities emerge regarding the obligation to carry out the second dose with vaccine in the under 60s to mRNA, already renamed as “heterologous” ». In fact, in the under 60s who received the first dose of AstraZeneca, the circular of 11 June 2021 of the Ministry of Health states that "the cycle must be completed with a second dose of mRNA vaccine (Comirnaty or Moderna)".
- Scientific evidence: Despite the immunological, biological and some historical precedents on the vaccine mix, the scientific evidence is still preliminary. In particular, the 4 studies cited by the CTS opinion enroll just over 800 people and measure the effectiveness of the mix only on the immune response and safety only on frequent and short-term side effects. In other words, to date there is no evidence of efficacy of "heterologous" vaccination on severe Covid-19, hospitalizations and deaths, nor on any rare side effects.
- Regulatory aspects. At the date of publication of the circular of the Ministry of Health, the vaccine mix appeared to be off label, i.e. outside the authorized indications. The AIFA determination of 13 June 2021 "remedied" the problem, with reference to law 648/96 and by providing that mRNA vaccines "can be administered as a second dose to complete a mixed vaccination cycle". That is, the possible formula used by AIFA to allow the use of "heterologous" vaccination contrasts with the peremptory one provided for by the circular of the Ministry of Health.
- Informed consent and professional responsibility. The reference to Law 648/96 provides for the "informed written consent of the patient showing that the same is aware of the incompleteness of the data relating to the safety and efficacy of the medicinal product for the proposed therapeutic indication". That is, the law 648/96 requires the citizen to accept or not the information provided to him (if he does not sign the consent he cannot complete the vaccination cycle) and to the doctor the responsibility of the prescription, in the presence of an alternative whose efficacy profile and safety was reiterated by the EMA.
«If immunological and biological assumptions and preliminary data – concludes Cartabellotta – suggest that the“ heterologous ”vaccination is effective and safe, there remains the inconsistency between the obligation envisaged by the circular of the Ministry of Health and the possibility reported by the Aifa determination. In fact, according to Aifa's formula for under 60s, the second dose with Pfizer or Moderna is only an option that the patient is free to accept or refuse, opting for the second dose with AstraZeneca. In any case, it is essential to adapt the informed consent form to the provisions of Law 648/96 with adequate information on the benefits, risks and uncertainties of the options for the second dose after AstraZeneca. Finally, to prevent the inconsistency between the expressions "duty" and "power" from translating into a responsibility exclusively borne by doctors, with the risk of discouraging vaccination, the Gimbe Foundation asks the Ministry of Health and the Aifa to express themselves jointly with a univocal and definitive note ».
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/cosa-non-va-nella-vaccinazione-eterologa-report-gimbe/ on Thu, 17 Jun 2021 08:06:52 +0000.
