Epochal study: the FDA evaluates the feasibility of the artificial womb
The US Food and Drug Administration (FDA) will hold a meeting of independent advisors on September 19 and 20. The agenda of the meeting is to discuss the feasibility of clinical trials using artificial womb technology to improve the survival and health of extremely premature newborns.
During this meeting, regulators and experts will delve into ethical concerns and evaluate various crucial aspects, including potential steps and the design of human trials for this technology, Nature reported .
Artificial womb for premature babies
A fertilized egg normally takes nine months to mature into a baby weighing around seven kilos.
However, complications can emerge along the path to welcoming a new life, potentially impacting the health and well-being of both the mother and the developing fetus. Infections, hormonal changes, hypertension and diabetes can make the uterus an unfavorable environment for the growing fetus.
This can result in premature births, occurring well before the normal length of a healthy pregnancy of about 40 weeks.
The current standard of care involves the use of incubators, which are used to keep premature babies warm and safe until their bodies reach a level of development sufficient to thrive outside the neonatal intensive care unit ( NICU) of the hospital.
With the rapid advancement of medical research and technology, artificial wombs are seen as the next big breakthrough.
This is a developing medical technique that aims to provide a controlled environment outside the womb for the gestation and growth of extremely preterm infants. The artificial womb technology will be specifically designed for babies born at less than 28 weeks gestation who may have a higher risk of death and morbidity.
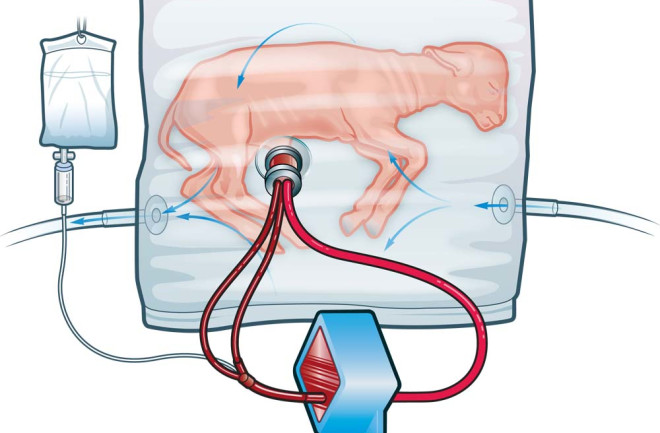
The artificial uterus tested in a lamb
The Children's Hospital of Philadelphia (CHOP) has reportedly asked for FDA approval to begin first-in-human clinical trials of an artificial uterus device called Extra-uterine Environment for Newborn Development, or EXTEND.
CHOP researchers established the feasibility of an artificial uterus called “Biobag” for raising a premature lamb in 2017.
The Biobag was infused with lab-created amniotic fluid, mimicking the natural liquid environment surrounding a fetus in the womb.
Other needs, such as a constant supply of oxygen, were introduced into the fetal bloodstream via a device connected to the umbilical cord.
The results showed that the lamb continued to grow inside the Biobag for up to four weeks after being removed from the natural womb, the equivalent of 23-24 weeks in a human pregnancy.
Here is a video showing the biobag in action
“Our system could prevent the severe morbidity experienced by extremely premature infants by potentially offering a medical technology that does not currently exist,” said Alan W. Flake, MD, a fetal surgeon at CHOP, in a 2017 press release.
Overall, the FDA briefing document mentions that valid human clinical trials for an AWT device must provide evidence to “support normal organ growth and maturation, while reducing the high rates of prematurity-associated morbidity seen with the current standard of care in the neonatal intensive care unit, in newborns born extremely premature.”
Even if at this stage it is still an aid for cases of illnesses in the mother's gestation, one has to wonder until this fertilization will be completely artificial, after it has recently been possible to create completely artificial embryos. Where are we going?

Thanks to our Telegram channel you can stay updated on the publication of new Economic Scenarios articles.
The article Epochal study: the FDA evaluates the feasibility of the artificial womb comes from Economic Scenarios .
This is a machine translation of a post published on Scenari Economici at the URL https://scenarieconomici.it/studio-epocale-la-fda-valuta-la-fattibilita-dellutero-artificiale/ on Tue, 19 Sep 2023 07:00:00 +0000.