Covid-19 vaccines. The Faq of the Ministry of Health
Covid-19 vaccines. The Faq of the Ministry of Health updated to 23 December 2020
What are the objectives of the COVID-19 vaccination campaign?
The goal of the population vaccination campaign is to achieve herd immunity to SARS-CoV2 as soon as possible. The campaign will start on December 27, given the approval by the EMA ( European Medicines Agency ) of the first COVID-19 vaccine. After an initial phase, which will have to be limited, due to the number of doses delivered, it will develop continuously increasing. Vaccines will be offered to the entire population, according to an order of priority, which takes into account the risk of disease, the types of vaccines and their availability.
Are vaccines safe?
Vaccines are only authorized after careful evaluation of the safety profile based on the studies carried out in the investigational phase.
In any case, the security profile will be continuously monitored even after authorization.
Will vaccination be mandatory?
At the moment it is not the Government's intention to make vaccination compulsory. During the campaign, the participation rate of citizens will be assessed.
What are the priority categories in the early stages?
- Health and social health workers. “Frontline” health and social health workers, both public and accredited private, have a higher risk of being exposed to COVID-19 infection and passing it on to susceptible and vulnerable patients in health and social settings. Furthermore, it is recognized that vaccinating frontline health and socio-health workers will help maintain the resilience of the health service.
- Residents and staff of residential care centers for the elderly. A high percentage of nursing homes (RSAs) have been severely affected by COVID-19. Residents of such facilities are at high risk of serious illness, due to advanced age, the presence of multiple comorbidities, and the need for assistance with eating and other daily activities.
- People of advanced age. An age-based vaccination schedule is generally easier to implement and allows for greater vaccination coverage. It is also evident that an age-based program increases coverage even in people with clinical risk factors, as the prevalence of comorbidities increases with age. Therefore, given the high probability of developing a serious disease and the consequent recourse to intensive or sub-intensive care admissions, this population group represents a priority for vaccination.
I fall into the categories to be vaccinated in the first phase, what should I do to access the vaccination?
In the first phase, vaccination will be reserved for health professionals, health and social health personnel of hospitals and local services as well as guests and staff of residential facilities for the elderly. These categories will be contacted with an active call.
I do not fall into the categories of people who are entitled to receive the vaccine in the first phase, when can I get the vaccine too?
The vaccination campaign will continue in phases that will depend on the quantity of vaccines available, on the indications of the EMA ( European Medicines Agency ) authorizations for each new vaccine and, in any case, will concern the population classes indicated in the Strategic Anti-Vaccination Plan. SARS-CoV-2 / COVID-19 .
The indications may be updated based on the evolution of the pandemic and the knowledge deriving from scientific research and the availability of vaccines.
At the moment these are the categories identified:
- health and social health personnel
- guests and staff of residential care centers for the elderly
- people aged 80 and over
- people who are 60 to 79 years old
- people of all ages who suffer from more than one previous chronic disease, immunodeficiency and / or disability.
Will children be vaccinated?
Comirnaty (Pfizer / Bionthec), the first vaccine approved by the EMA (European Medicines Agency), is currently not recommended for children under the age of 16. The European Agency, as well as other international agencies, await further studies in order to authorize vaccination on the pediatric population.
Will immunosuppressed people be vaccinated?
Limited data are available in people with immunodeficiency or being treated with immunomodulatory drugs. Although such individuals may not respond as well to the vaccine, there are no particular safety concerns. According to the Strategic Plan, people with immunodeficiency or being treated with immunomodulating drugs will have to be vaccinated in the early stages, as they are more susceptible to get sick with COVID-19.
Will people who have already had COVID-19 be vaccinated?
Yes, those who have had COVID-19 can be vaccinated.
Who controls the adverse reactions? Who should they be communicated to? And who evaluates them?
AIFA (Italian Medicines Agency), in addition to the pharmacovigilance activities normally provided for drugs and vaccines (based on spontaneous reports and on the pharmacovigilance networks already present), will promote the launch of some independent post-authorization studies on COVID vaccines. 19.
The supervisory activities will concern both the collection and evaluation of spontaneous reports of suspected adverse reactions (passive pharmacovigilance) and proactive actions, through pharmaco-epidemiology studies / projects (active pharmacovigilance). AIFA has a Scientific Committee which, for the entire period of the vaccination campaign, will have the function of supporting the Agency and the scientific managers of the individual studies in the phase of setting up the activities, in the overall analysis of the data that will be collected and in the identification of possible interventions. The aim is to have, also through an international collaborative network, the ability to highlight any possible risk signals and, at the same time, to compare the safety profiles of the different vaccines that will become available, to provide recommendations.
What are the objectives of the COVID-19 vaccination campaign?
The goal of the population vaccination campaign is to achieve herd immunity to SARS-CoV2 as soon as possible. The campaign will start on December 27, given the approval by the EMA (European Medicines Agency) of the first COVID-19 vaccine. After an initial phase, which will have to be limited, due to the number of doses delivered, it will develop continuously increasing. Vaccines will be offered to the entire population, according to an order of priority, which takes into account the risk of disease, the types of vaccines and their availability.
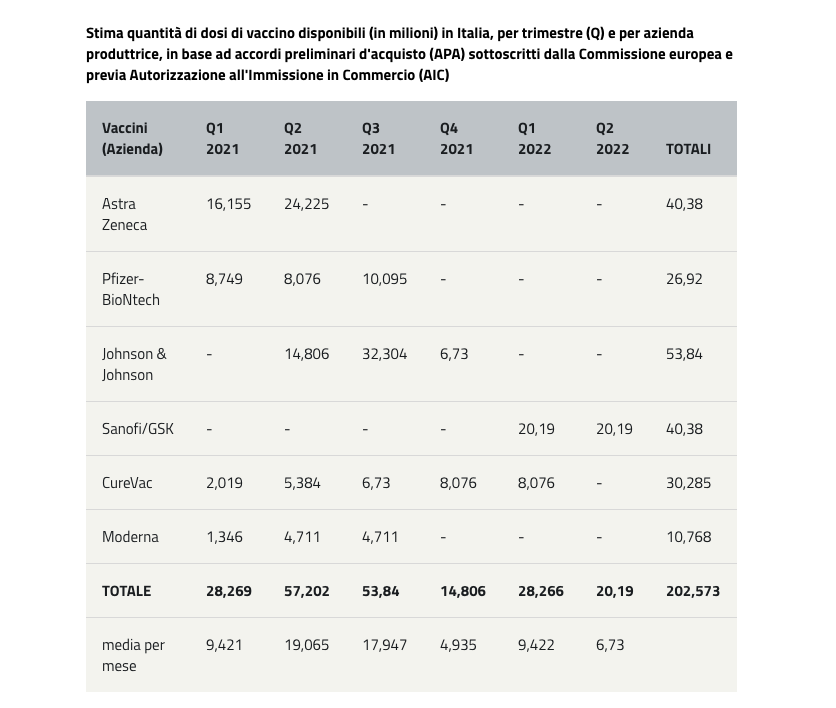
Which vaccines will arrive in Italy and when?
The timing and figures may be subject to variations depending on the authorization and dose assignment processes.
According to the agreements signed, Italy will be able to count on the availability of the following doses:
How many doses do you need to be immunized?
For almost all vaccines close to authorization at the moment, two doses are scheduled a few weeks apart depending on the type of vaccine.
Is there a provision for an international vaccination certificate?
A normal vaccination certificate will certainly be issued.
International institutions such as the European Commission and WHO are evaluating a proposal for an international digital certificate.
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/vaccini-anti-covid-19-le-faq-del-ministero-della-salute/ on Sat, 26 Dec 2020 17:00:31 +0000.
