Here are the details on the Pfizer-Biontech vaccine (approved by the EMA)
Ema has recommended conditional authorization of the vaccine for Covid-19 developed by Pfizer / Biontech. All the details on the decision and the data published so far on the effectiveness of the vaccine
Green light also from the European Union to the anti Covid vaccine developed by Pfizer-Biontech. "The EMA has recommended the conditional authorization of the vaccine for Covid-19 developed by Pfizer / Biontech", announced Marie-Agnes Heine, director of communication of the European Medicines Agency who gave the green light to the vaccine against the coronavirus developed by BioNTech and Pfizer.
The vaccine, also authorized for use by regulatory authorities in Great Britain, the United States and Switzerland, is 95% effective. Let's go step by step.
EMA APPROVES FIRST ANTI COVID DRUG
We have a first drug against Covid-19 authorized for use in the European Union: it is the vaccine developed by the American Pfizer with the collaboration of the German Biontech. Ema, announced Marie-Agnes Heine, recommended conditional authorization of the vaccine.
“The authorization of the Pfizer / BioNTech vaccine is a significant step forward in our fight against this pandemic that is causing suffering not only in Europe but around the world,” said Emer Cooke, executive director of EMA. "In less than a year, an authorized vaccine has been reached, which is the result of unprecedented work," he added.
TOWARDS THE MARKETING
The scientific opinion is the first step for the marketing of the anti Covid vaccine in the EU, "with all the guarantees, controls and obligations that this entails", reads a note.
VACCINE FROM 16 YEARS OLD
And the decision regarding a possible commercialization and therefore the start of the vaccination campaign could arrive already tonight, according to what was announced by the president Ursula van der Leyen.
The Pfizer-Biontech vaccine "can be used from the age of sixteen" in the 27 countries of the European Union, said the executive director of the EMA, Emer Cooke at a press conference. Vaccinations could start on January 27.
PREGNANT WOMEN: CASE-BY-CASE ASSESSMENT
For pregnant women, however, it will be necessary to evaluate case by case. “For pregnant women we recommend a case-by-case approach based on the individual situation and considering the risk of exposure and infection,” explained Harald Enzmann, chairman of the EMA committee.
The tests conducted, explains Enzmann, "did not involve enough women during pregnancy", but, "we also implemented initiatives to fill this knowledge gap as soon as possible".
THE SIDE EFFECTS
What are the unwanted side effects? For Ema there is no need to worry, despite being an mRna vaccine: "the side effects of this vaccine are the same as those found on other vaccines," said Sabine Straus, president of the Ema safety committee. . You may experience "pain during the injection phase, fatigue, headache, pain in the muscles and ligaments, high fever", but "they last about a day", explained the expert who nevertheless recommended "an important action of monitoring ”to the health authorities on the effects found.
THE VACCINE AND THE COVID VARIANT
Will the vaccine also be effective against the "Covid variant"? EMA will continue to analyze data. "We know a lot more about this vaccine than we did ten months ago and even three months ago, but there is still new information that needs to be evaluated in its entirety, such as recent information on the new coronavirus variant that still needs to be evaluated," Emer Cooke said.
"In the meantime we must do our best to prevent" Covid-19 "following the directions of the health authorities: wear masks, wash your hands and keep your distance," added Cooke.
THE PFIZER-BIONTECH VACCINE
The vaccine developed by the American Pfizer and the German Biontech, BNT162b2, is based on the glycoprotein (S) spike antigen of SARS-CoV-2 encoded by Rna and formulated in lipid nanoparticles (LNP).
The vaccine must be stored at a temperature between minus 80 and minus 60 degrees and needs to be diluted once thawed.
I STUDY
The vaccine has been tested in the USA, Argentina, Brazil, Germany, South Africa and Turkey on approximately 44,000 volunteers. The randomized, double-blind study involved 21823 participants who were given the vaccine and 21828 volunteers who were given placebo.
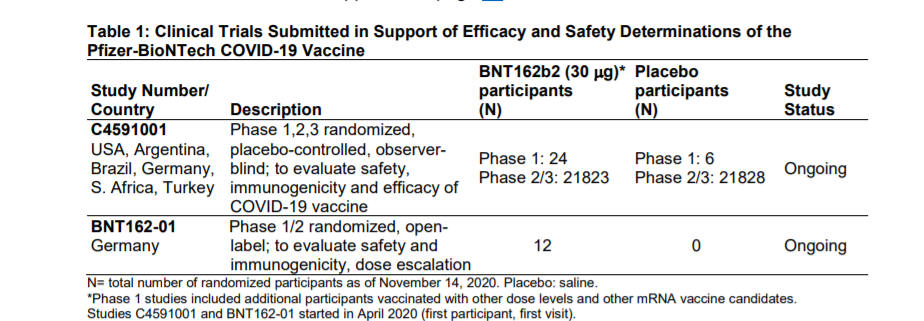
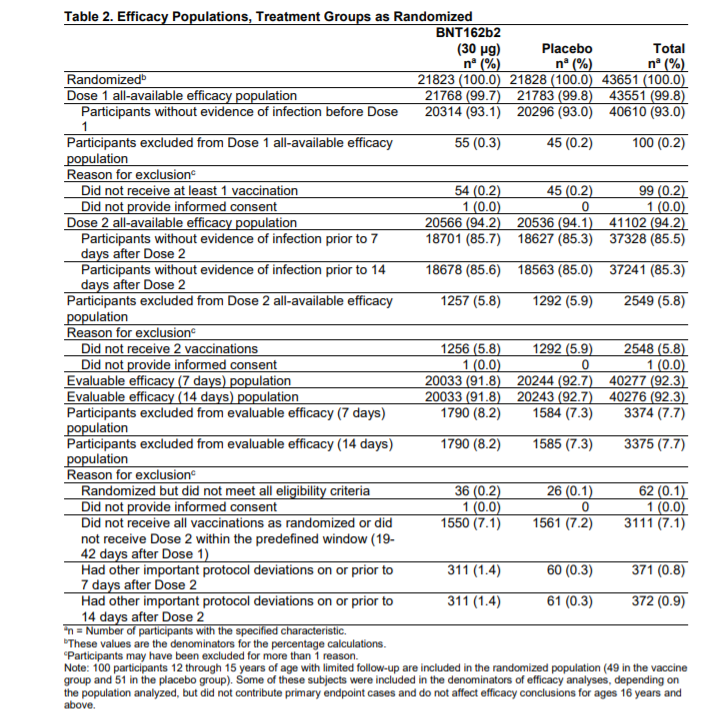
THE EFFECTIVENESS OF THE PFIZER-BIONTECH VACCINE
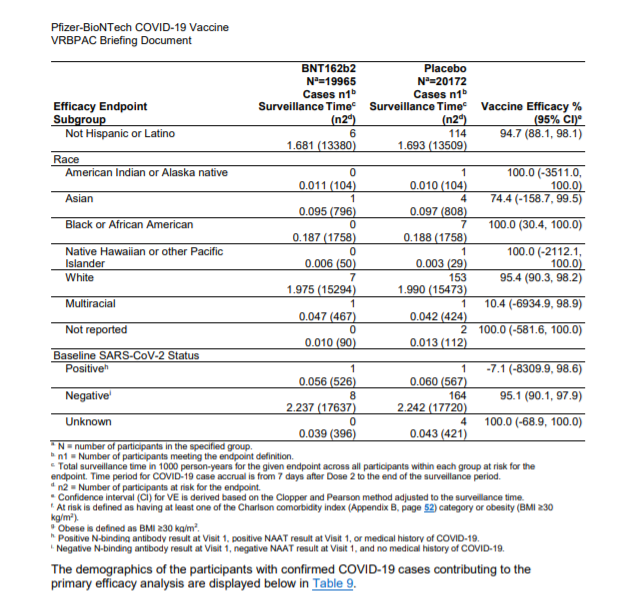
The vaccine was shown to be 95% effective 7 days after the second dose. In particular, it has a protection efficacy against Covid-19 of 95.6 among those between 16 and 65 years and an efficacy of 83.7 among those over 55.
IMMUNIZATION
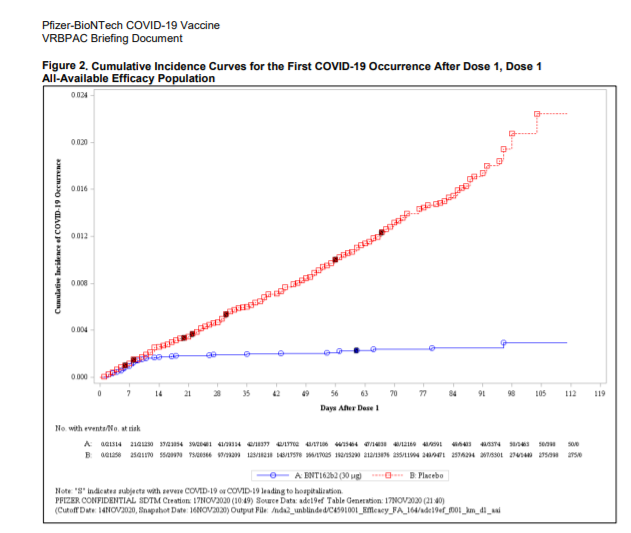
The vaccine, as Roberto Burioni explains in Medical Fatcs , keeps its promises of efficacy. "Not only from the twelfth day (coincidentally, it takes about 14 days for our body to produce antibodies …) we see a protective effect, but in this period of time there have been four severe Covid-19s, but all in unvaccinated . It could be due to the presence of small amounts of antibodies which, however, are able to make the disease milder, exactly as happens with the varicella vaccine, which if done during incubation does not completely inhibit the disease, but makes it much more mild ".
A STRONG NEUTRALIZATION
The efficacy demonstrated by Pfizer's drug is therefore very high. “The vaccine managed to go beyond the human reaction. We have seen much stronger neutralization of much better performing antibodies. Only one other vaccine has this "super human" power, which is papilloma. The answer is better than what we could have anticipated ”, commented Eric Topol, director of the Scripps Research Translational Institute at Che tempo che fa, a broadcast of Rai 3 conducted by Fabio Fazio. In practice, the vaccine is more potent than the disease in inducing immunization.
A TURNING POINT FOR THE FUTURE
But the effectiveness of Pfizer's vaccine, the first ad mRna to be approved in history, is not only a victory (hopefully) against Covid-19, but also for any future pandemics. “Even if it all happened in 11 months – said Topol – this is the result of 10 years of research, now we have a new platform. We can say that we are ready for the next pandemic ”.
This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/vaccino-di-pfizer-biontech-ema/ on Mon, 21 Dec 2020 08:39:23 +0000.