What is written on the Astrazeneca vaccine form

Informed consent to the Astrazeneca vaccine: what is written on the form for vaccination against Covid 19 with Astrazeneca
Before the inoculation of the Covid-19 vaccine, the "Consent Form for the vaccination against Covid 19 of the general population" must be signed. It is different for each drug that needs to be administered.
What does that of Astrazeneca, which in recent weeks is being administered to teachers and law enforcement officers?
Here is the information taken from the form published on the website of the Ministry of Health.
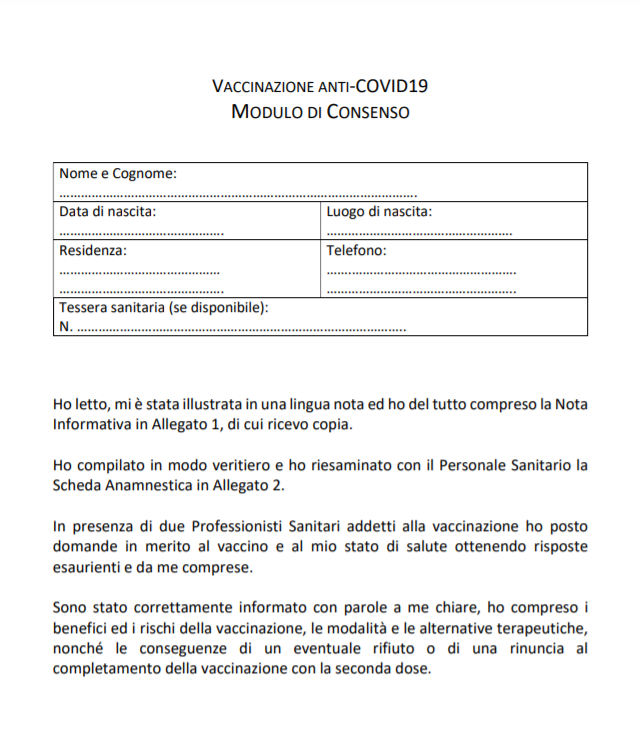
CONSENT
After declaring that he has read the Information Note, the patient undertakes that in the presence of side effects he will inform "immediately" his doctor and agrees and authorizes "the administration of vaccination using the 'COVID-19 Vaccine AstraZeneca' vaccine".
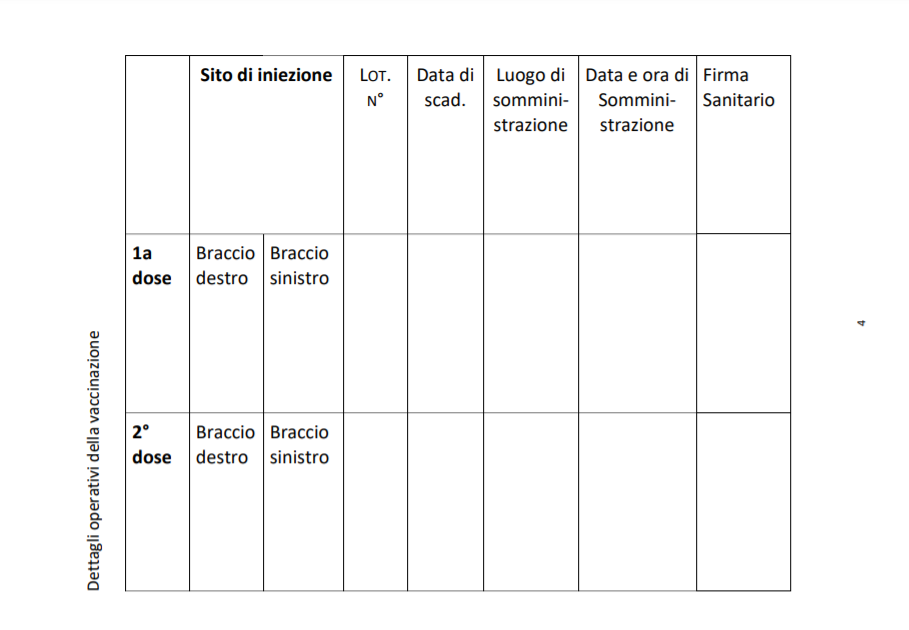
THE ADMINISTRATION
The vaccine "requires 2 doses, 4-12 weeks (28 to 84 days) apart". It will be the healthcare operator's responsibility to indicate the method and place of inoculation.
LIMITED EFFECTIVENESS ABOVE 55 YEARS
The annex to the consent form clarifies that the vaccine "is administered to adults aged 18 years and over" and that "limited data are currently available on the efficacy of 'COVID-19 Vaccine AstraZeneca' in subjects aged 18 or over 55 ".
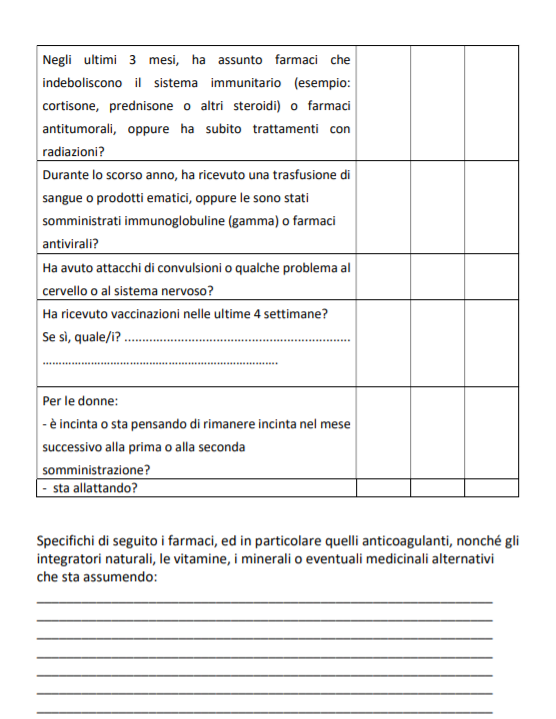
PREGNANT WOMEN
There is little evidence on efficacy and side effects even for pregnant women. “On the basis of current scientific knowledge, the administration of the vaccine cannot be recommended or contraindicated to pregnant and lactating women. The administration of the vaccine can only be carried out after the analysis, case by case with the reference health professional figure, of the potential risks and potential benefits for the mother, the fetus and the newborn. It is not known whether 'COVID-19 Vaccine AstraZeneca' is excreted in human milk ”.
THE PROTECTION
In general, the protection of the vaccine against Covid-19 begins "from about 3 weeks after the first dose", but the "vaccinated may not be fully protected until 15 days after the administration of the second dose".
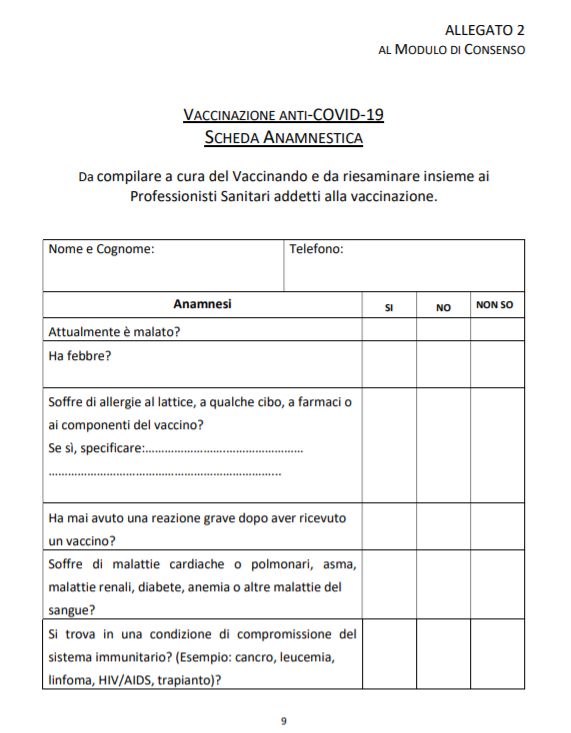
ADVERSE REACTIONS
The consent form also explains the adverse reactions. The very common ones (including, tenderness, warmth, itching or bruising where the injection is given; feeling tired or generally unwell; chills or feeling of fever; headache; nausea); common ones (swelling or erythema where the injection is given; fever; vomiting or diarrhea); less common ones (sleepiness or dizziness; decreased appetite; swollen lymph nodes; sweating, itching or rash).
ALLERGIC REACTIONS
Serious symptoms (including, feeling faint or lightheaded; changes in heartbeat; shortness of breath; wheezing), "which may be related to an allergic reaction", are not excluded.








This is a machine translation from Italian language of a post published on Start Magazine at the URL https://www.startmag.it/sanita/che-cosa-e-scritto-sul-modulo-per-il-vaccino-di-astrazeneca/ on Tue, 02 Mar 2021 10:45:27 +0000.
